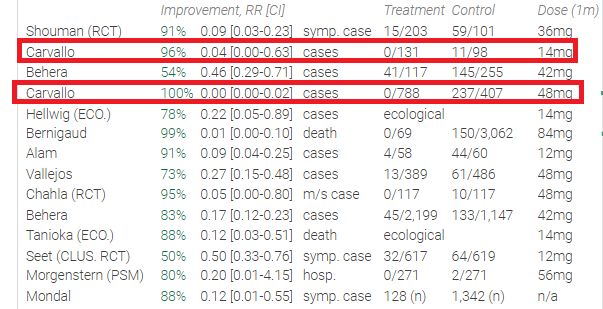
People commonly claim that pharmaceutical companies can't make money on drugs that are off or close to being off-patent
So let's talk about the famous case of Nexium and Prilosec 1/n
So let's talk about the famous case of Nexium and Prilosec 1/n
2/n The story begins in 1989, when the company that later became Astrazeneca patented a drug for acid reflux that worked better than anything on the market. The drug, named Prilosec, became one of the best-selling medications at the time
3/n However, in the late 90s/early 00s, the company making Prilosec faced an issue - their drug was soon to be off patent, and despite record sales generic manufacturers wanted to start selling cheaper versions 

4/n After stalling generic manufacturers for several years, Astrazeneca came out with a new drug for the same problem, Nexium, which they claimed had significant advantages over Prilosec
Nexium quickly became one of the highest-earning drugs of all time
Nexium quickly became one of the highest-earning drugs of all time

5/n However, there were some issues. While the manufacturer claimed that Nexium was way better, studies run comparing the two drugs found little to no benefit 

6/n While there were some demonstrable benefits for Nexium over Prilosec, they were for much less common conditions than the main indication, which definitely wouldn't justify the sales
7/n This was perhaps unsurprising, because of a key fact - Nexium and Prilosec are two similar molecules. The generic names give this away quite clearly - omeprazole is Prilosec, and Nexium is called...esomeprazole
8/n When I say similar, I don't mean "vaguely the same". What I mean is that esomeprazole is one of two isomers present in omeprazole. Here's what they look like side-by-side 



9/n Being one isomer of the molecule, esomeprazole is so similar as to be basically identical. While isomers can have very different properties, in this case omeprazole contains both isomers so the difference, if any, is largely academic chemistry-blog.com/2010/10/18/nex…
10/n In other words, the industry took a molecule that was coming off patent, split off one version of that molecule, patented that, and made it the best-selling drug of all time even though the evidence for benefit was...pretty slim
11/n Now, this sounds like a scoop, and it would be except that the pharmaceutical industry was, well, proud of doing this. Here's an article that describes the process, with pharma execs talking about how great the project went wsj.com/articles/SB102…
12/n The whole story is amazing, but some choice moments include the name of the whole thing - Shark Fin - and the amazing story of how the company managed to keep Prilosec under patent just long enough 





13/n But the point is, this wasn't some shady plan. The company was quite proud of their drug, proud of the sales, and proud of their assessment that it was better. They even marketed Nexium identically to Prilosec, down to the pill colour!
14/n It's perhaps the most famous example of biosimilars, and it's not some vague similarity in method of action but two drugs that are arguably identical, except for over a decade one cost 10x more than the other 🤷♂️
15/n A useful read if you're interested in this topic is this article which itself has a great book referenced
ncbi.nlm.nih.gov/pmc/articles/P…
ncbi.nlm.nih.gov/pmc/articles/P…
16/n And finally, for those still skeptical, while Nexium sales have declined since the drug came off patent in 2015, it appears that globally (according to Statista) the drug is still earning AZ well over a billion dollars a year 

17/n Anyway, for those still reading, if pharma wanted to make money off ivermectin they easily could. It's not like they haven't done it before!
Instead, they've publicly disavowed the drug and said it probably doesn't work.
Instead, they've publicly disavowed the drug and said it probably doesn't work.
• • •
Missing some Tweet in this thread? You can try to
force a refresh