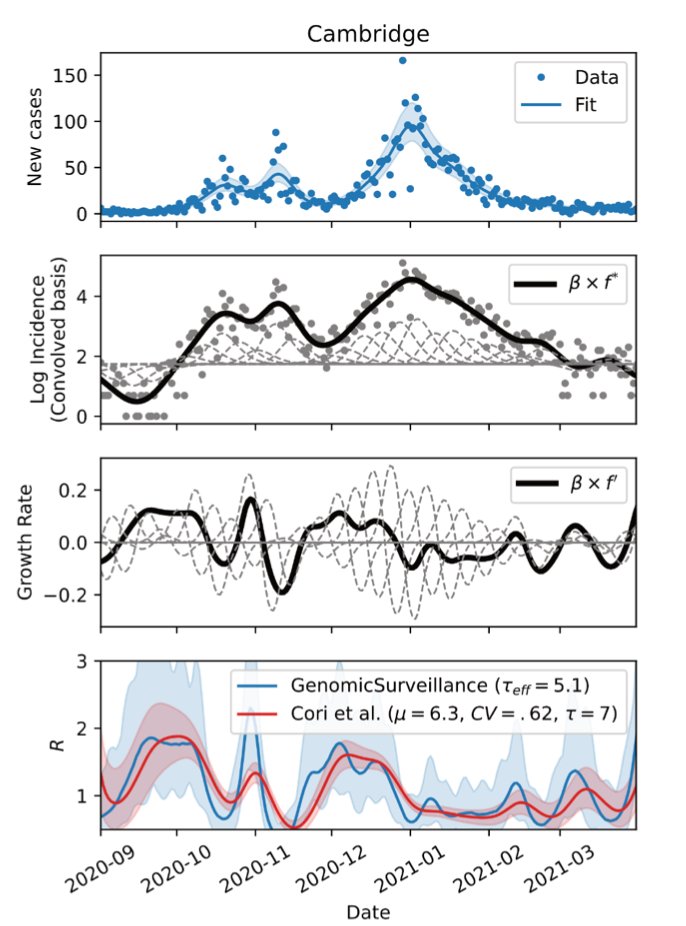
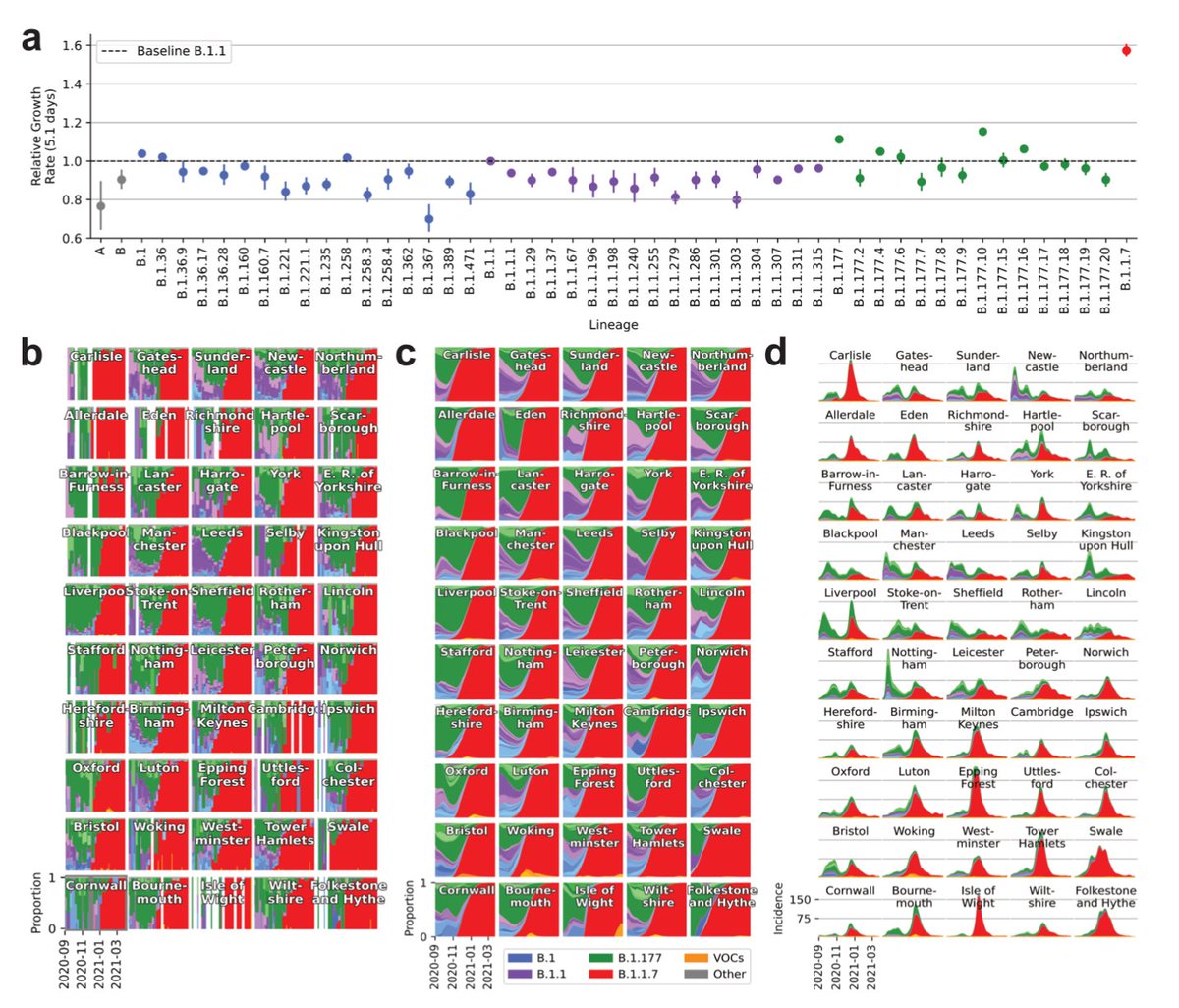
Some thoughts about the punctuated evolution of variants of concern including B.1.1.529 in Southern Africa. 🧵
A shared characteristic of all known VOCs is that they appeared suddenly with a large number of mutations, many more than the incremental changes we see normally.
A shared characteristic of all known VOCs is that they appeared suddenly with a large number of mutations, many more than the incremental changes we see normally.

These mutations recurrently cluster in certain functional sites of the virus’ genome.
This is the signature of selection — while mutations occur more or less randomly, we preferentially see the subset that makes the virus fitter.
But why so many concomitant mutations at once?
This is the signature of selection — while mutations occur more or less randomly, we preferentially see the subset that makes the virus fitter.
But why so many concomitant mutations at once?
https://twitter.com/miamalan/status/1463846528578109444
While we will never know the exact circumstance of each VOC emergence, we do know that a similar pattern occurs in immunocompromised patients who have chronic infections.
This includes patients with leukaemia, but also AIDS
medrxiv.org/content/10.110…
nature.com/articles/s4158…
This includes patients with leukaemia, but also AIDS
medrxiv.org/content/10.110…
nature.com/articles/s4158…
The theory is that months-long infection acts like an incubator, which allows the virus to assemble a large armamentarium of advantageous mutations.
It is possible that the fitness effect of mutations is not one-by-one, but jumps once the right constellation of mutation occurs.
It is possible that the fitness effect of mutations is not one-by-one, but jumps once the right constellation of mutation occurs.
Variants with long branches have repeatedly emerged in Southern Africa. They include B.1.351 (Beta), C.1.2, B.1.1.528 and now B.1.1.529.
The regional clustering and characteristics of variants suggests that there is a large reservoir of chronic infection.
The regional clustering and characteristics of variants suggests that there is a large reservoir of chronic infection.
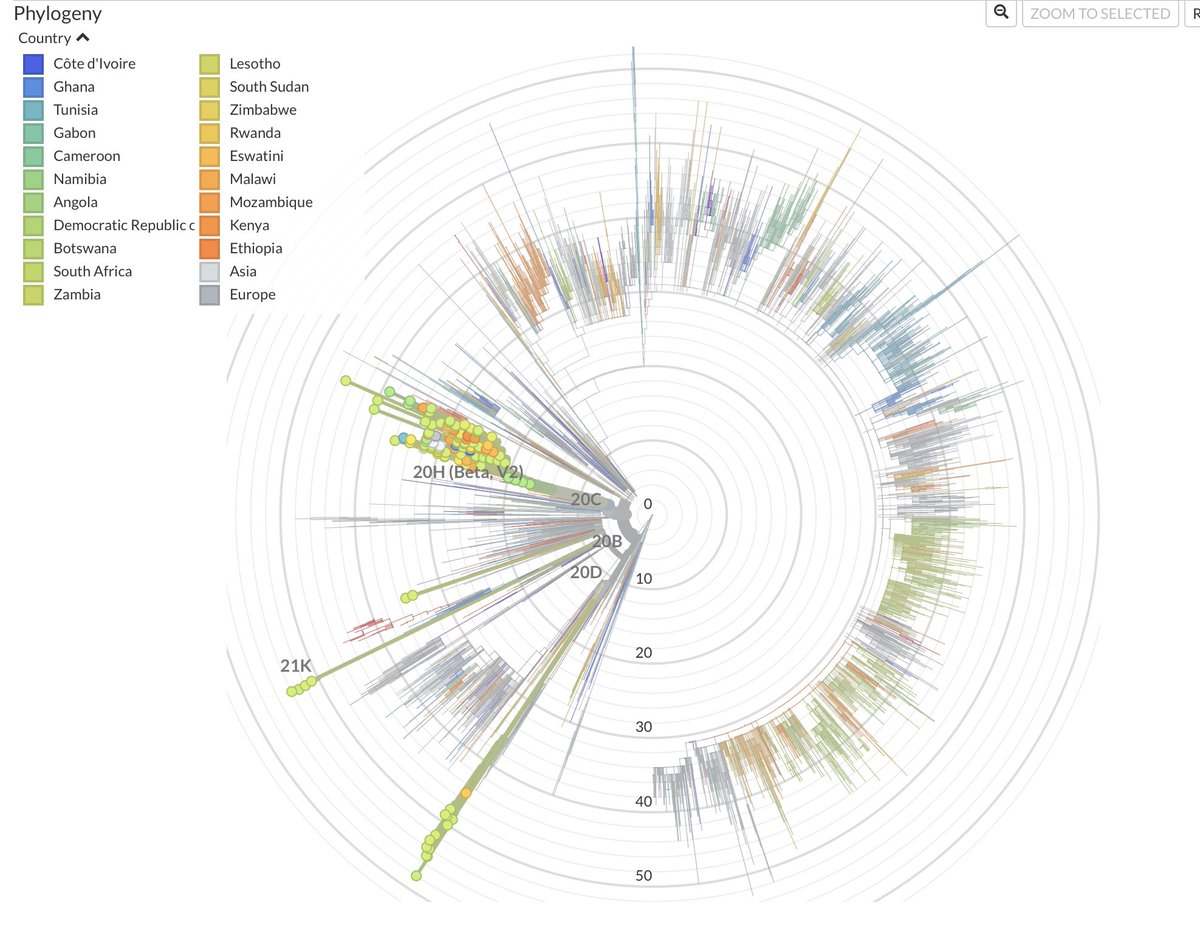
Often ignored by the rest of the world, Southern Africa has a large HIV epidemic, with around 20% of the population infected.
Despite large national government efforts, only 71% of infected people in South Africa have access to antiretroviral therapies.
unaids.org/en/regionscoun…
Despite large national government efforts, only 71% of infected people in South Africa have access to antiretroviral therapies.
unaids.org/en/regionscoun…
The regional emergence of several SARS-CoV-2 variants with long branches and concerning mutations in Southern Africa suggests that its HIV epidemic is a driving factor.
This is a factor that the global pandemic response may have to acknowledge.
This is a factor that the global pandemic response may have to acknowledge.
I don’t think efficacy data is available, but SARS-CoV-2 vaccination elicits strong immune responses in HIV patients that undergo antiretroviral therapies.
thelancet.com/journals/lanhi…
thelancet.com/journals/lanhi…
In order to prevent chronic infections in people living with HIV, a combination of HIV antiretroviral therapy and SARS-CoV-2 vaccination seems necessary.
That failing, antiviral SARS-CoV-2 therapy may be required to stop chronic infections.
That failing, antiviral SARS-CoV-2 therapy may be required to stop chronic infections.
The international pandemic response may therefore have to look not only at providing equitable access to SARS-CoV-2 vaccines and financial support for public health systems — but also into HIV and SARS-CoV-2 antiviral therapies.
Nobody is safe until everyone is safe.
Nobody is safe until everyone is safe.
• • •
Missing some Tweet in this thread? You can try to
force a refresh