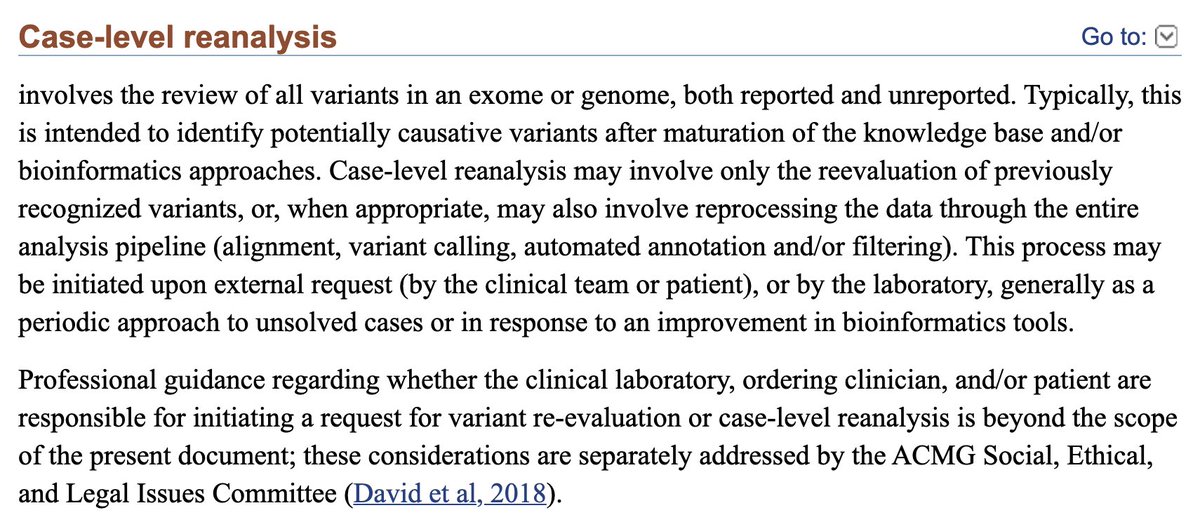
@MJLBio @Sanctuary_Bio @Biohazard3737 Sure! I realize I was being a little vague with those statements. Generally, I think you're correct in your interpretation of the importance of P2 (great $/GB, but at a smaller scale) as well as duplex sequencing.
Something that is important to recognize, though ...
Something that is important to recognize, though ...
@MJLBio @Sanctuary_Bio @Biohazard3737 ... is how product deployment works differently between PacBio and Nanopore, which is partly an artefact of culture and of time in the public markets, in the public markets. I'm not advocating for one over the other with my next statements.
@MJLBio @Sanctuary_Bio @Biohazard3737 PacBio has been a public company for a long time. While the management has changed much since the failed Illumina merger, the familiarity with how to operate as a public company has not.
PacBio is more secretive and only unveils fully built-out commercial products.
PacBio is more secretive and only unveils fully built-out commercial products.
@MJLBio @Sanctuary_Bio @Biohazard3737 When new products come out, the advancements are typically orders-of-magnitude better.
Nanopore has a very transparent, quick-turn deployment schedule that is all out in the open. The improvements are visible/incremental, which is a great value proposition for its customers.
Nanopore has a very transparent, quick-turn deployment schedule that is all out in the open. The improvements are visible/incremental, which is a great value proposition for its customers.
@MJLBio @Sanctuary_Bio @Biohazard3737 To reiterate, I'm not saying one is better than the other, but undoubtedly they are different.
• • •
Missing some Tweet in this thread? You can try to
force a refresh