It is this time of the year again: TOP 20 GU Oncology clinical trials published in 2021:
Some negative, some positive, some led to @FDAOncology approval; we learned from all!
Feel free to add & retweet & tag anyone involved
This is not an exhaustive list
@OncoAlert
Some negative, some positive, some led to @FDAOncology approval; we learned from all!
Feel free to add & retweet & tag anyone involved
This is not an exhaustive list
@OncoAlert

1/ (177Lu)–PSMA-617 showing improved PFS and OS in pre-treated mCRPC patients vs. SOC, in addition to a favorable safety profile @sartor_oliver @morr316 @NEJM
nejm.org/doi/full/10.10…



nejm.org/doi/full/10.10…




2/ MRI with targeted & standard biopsy in men with MRI suggestive of prostate cancer (PC) was noninferior to standard biopsy for detecting clinically significant PC &resulted in less detection of clinically insignificant cancer @NEJM @TobiasNordstrom
nejm.org/doi/full/10.10…


nejm.org/doi/full/10.10…



3/ ACIS Phase 3 trial: Combo apalutamide + abiraterone (vs. abiraterone/placebo) in patients with mCRPC => improved rPFS & an acceptable safety profile @DRathkopf @EfstathiouEleni @AttardLab @PCFnews #FredSaad @chumontreal @TheLancetOncol
thelancet.com/article/S1470-…

thelancet.com/article/S1470-…


4/ TRANSFORMER phase 2 trial showing a meaningful clinical activity of bipolar androgen therapy (BAT) in the management of mCRPC, along with acceptable safety profile @EAntonarakis @neerajaiims @sonpavde @MarkowskiGUOnc #JorgeGarcia ascopubs.org/doi/full/10.12… 





5/ IPATential 150 phase 3 trial @TheLancet with combination of ipatasertib and abiraterone (vs. abiraterone + placebo) showing improved rPFS in mCRPC and functional PTEN-loss (not in ITT pop) @ChrisSweens1 @DanaFarber_GU #deBono @drcmassard
thelancet.com/journals/lance…

thelancet.com/journals/lance…


6/ The POP-RT phase 3 trial showing improved 5-year biochemical failure free survival (BFFS) and DFS with whole-pelvic radiotherapy (WPRT) vs. prostate-only radiotherapy in patients with high risk #PCa @VedangMurthy @docpriyamvada
ascopubs.org/doi/abs/10.120…


ascopubs.org/doi/abs/10.120…



7/ The CheckMate 274 phase 3 trial showing improved DFS with adjuvant nivolumab (vs. placebo) in patients with MIBC who had undergone surgery @MattGalsky @IcahnMountSinai #DeanBajorin @sloan_kettering @DrShariat nejm.org/doi/full/10.10… 





8/ Phase 3 EV-301 trial showing improved PFS and OS with EV (vs. chemo) in patients with previously treated mUC @tompowles1 @Bartscancer @DrRosenbergMSK @sloan_kettering @DanielPetrylak @YaleCancer @cdanicas @y_loriot @sonpavde @DanaFarber_GU nejm.org/doi/full/10.10… 





9/ Pembrolizumab monotherapy showing promising activity in patients with BCG-unresponsive NMIBC with CR rate of 41% at 3 months and an acceptable safety profile @ArjunBalarMD @UroDocAsh @PGrivasMDPhD @bkonety @eric_facs thelancet.com/journals/lanon… 



10/ The phase 3 KEYNOTE-361 trial showing that the addition of pembrolizumab to first-line platinum-based chemotherapy does not improve PFS/OS of patients with mUC @tompowles1 @AlvaAjjai @UMRogelCancer @y_loriot @GustaveRoussy
thelancet.com/journals/lanon…


thelancet.com/journals/lanon…



11/ Phase 3 IMvigor010 trial of adjuvant atezolizumab in muscle-invasive urothelial carcinoma showing no major improvement in outcomes/DFS as compared to observation @OncoBellmunt @df_hcc @BIDMChealth @siadaneshmand #MahaHussain @NorthwesternU
thelancet.com/article/S1470-…

thelancet.com/article/S1470-…


12/ The phase 2 study of neoadjuvant gemcitabine + cisplatin + pembrolizumab pre- cystectomy in MIBC: met its primary endpoint for improved pathologic downstaging @TracyLRoseMD @MRHarrisonMD @MattMilowsky @WilliamKimMD @TiansterZhang @angiesmith_uro ascopubs.org/doi/abs/10.120… 



13/ The phase 2 trial TALAPRO-1 showing durable anti-tumor activity of talazoparib monotherapy in heavily pretreated patients with mCRPC @NivenMehra #DeBono #Fizazi @jodebon @ICR_London @JCO_ASCO ascopubs.org/doi/abs/10.120… 







14/ TROPHY-U-01, evaluating the efficacy and safety of SG in patients with mUC who progressed on platinum or checkpoint inhibitors with ORR=27.4%, and manageable AEs @DrScottTagawa @PGrivasMDPhD @ArjunBalarMD
ascopubs.org/doi/full/10.12…

ascopubs.org/doi/full/10.12…


15/ Phase 3 CALGB 90601 trial of gemcitabine + cisplatin + bevacizumab showing no improved OS compared to gem/cis only in 1L patients with mUC @DrRosenbergMSK @morr316 @ERPlimackMD @ChrisSweens1 @ALLIANCE_org @WalterStadler5 @DrScottTagawa ascopubs.org/doi/abs/10.120… 



16/ Phase II trial of HIF-2α inhibitor Belzutifan showing promising results in patients with VHL disease with renal (RR=49%) and non-renal (RR=77% in pancreas, 30% in CNS) neoplasms @EJonasch @theNCI @FDonskov #Srinivasan #Linehan @KimrynRathmell @NEJM nejm.org/doi/full/10.10… 



17/ Phase 3 KEYNOTE-564 trial comparing adjuvant pembrolizumab to placebo in patients with ccRCC at int/high risk for recurrence after nephrectomy is + for DFS @tompowles1 @HHammersMD #NaomiHaas @ASCO @myESMO @AmerUrological, COI: 1st author
nejm.org/doi/full/10.10…

nejm.org/doi/full/10.10…


18/ The three-arm phase 3 CLEAR trial of pembro + lenv vs. lenv. + everolimus vs. sunitinib in patients with mRCC showing longer PFS/OS/ORR with pem/len group vs. sunitinib @motzermd @NEJM @schmidingerRCC @ViktorGruenwald; COI: senior author
nejm.org/doi/10.1056/NE…


nejm.org/doi/10.1056/NE…



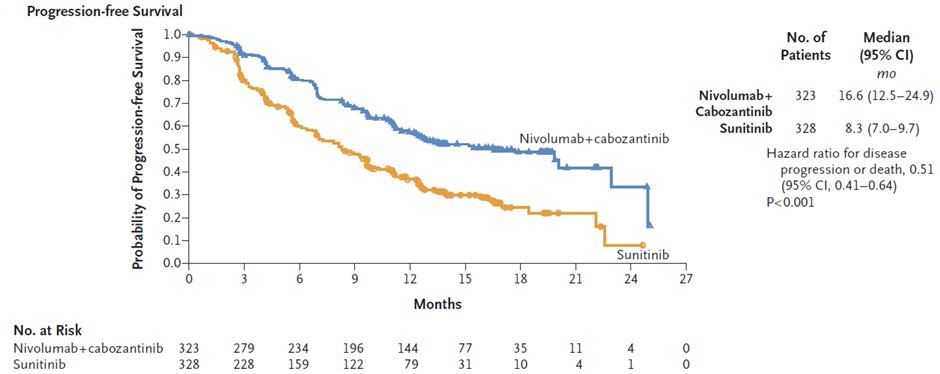
19/ Phase 3 CheckMate-9ER trial of nivolumab+cabozantinib vs. sunitinib in 1L metastatic ccRCC showing improved PFS/OS/ORR for nivo + cabo across subgroups @motzermd @tompowles1 @apolo_andrea @BourlonMaite #JensBedke @uni_tue; COI: 1st author
nejm.org/doi/full/10.10…


nejm.org/doi/full/10.10…


20/ Randomized trial @SWOG 1500 trial in advanced papillary RCC of cabozantinib, sunitinib, crizotinib and savolitinib. Cabo showed benefit in PFS (9mo)/ RR (23%) vs. sunitinib (5.6mo, 4%) @montypal @PrimoLaraMD @DrDanielHeng @Daniel_J_George @TheLancet
thelancet.com/journals/lance…

thelancet.com/journals/lance…


I am sure many more studies are out there; but this is a collection from top @ASCO @myESMO @JCO_ASCO @TheLancetOncol @NEJM @TheLancet @Twitter #plenaries @OncoAlert @Uromigos @OncLive + many more. Stay safe and to a healthy 😷and successful 2022.
Fin...
Fin...
• • •
Missing some Tweet in this thread? You can try to
force a refresh