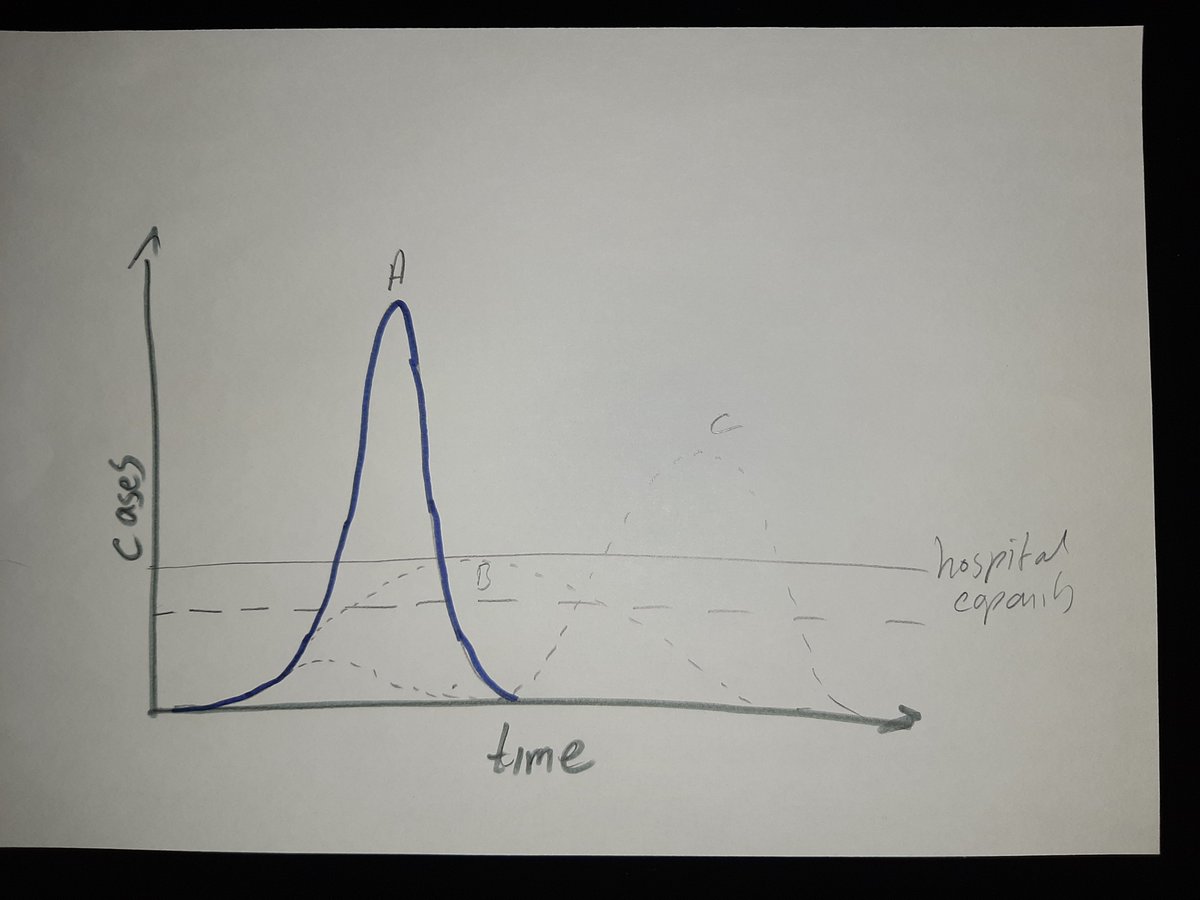
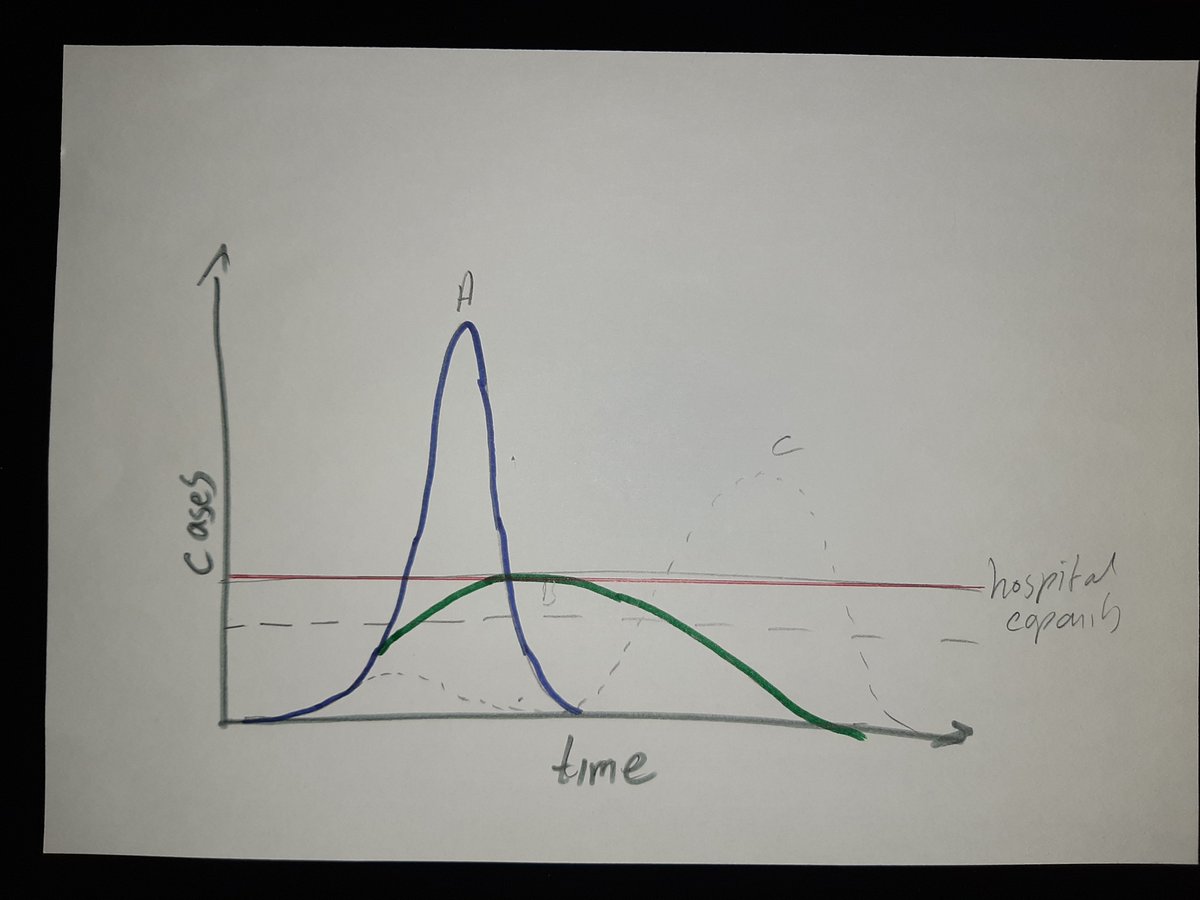
A thread on trust in vaccines.
Or why I think it is wrong to say say "we can't know vaccines are safe, because just look how long it took to discover the harm from smoking, x-rays, asbestos".
/

Or why I think it is wrong to say say "we can't know vaccines are safe, because just look how long it took to discover the harm from smoking, x-rays, asbestos".
/


The "it is too soon to know it is safe" argument initially seems quite rational, and is the foundation of much vaccine hesitancy and widespread in anti-vaccine literature.
I don't want to focus on rebutting claims about the science, but to consider process and implications.
/
I don't want to focus on rebutting claims about the science, but to consider process and implications.
/
There are two levels of protection we as members of the public have against unsafe vaccines.
1. medicines regulators (FDA, EMA, MHRA etc) that determine if they are safe and effective
2. vaccine authorities that decide whether universal vaccination is justified.
/
1. medicines regulators (FDA, EMA, MHRA etc) that determine if they are safe and effective
2. vaccine authorities that decide whether universal vaccination is justified.
/
That first line of defence is regulators.
They apply very strict rules to prevent companies selling vaccines (and other medicines) without compelling data on efficacy and safety, collected according to a pre-agreed protocol, with very high quality of documentation.
/
They apply very strict rules to prevent companies selling vaccines (and other medicines) without compelling data on efficacy and safety, collected according to a pre-agreed protocol, with very high quality of documentation.
/
None of the hazardous products used to justify long term vaccine risk (cigarettes, x-rays, asbestos) went through any pre-market process They also all involve long term exposure (unlike vaccines).
So what?
Well lets look at why we have medicine regulators and how they work
/
So what?
Well lets look at why we have medicine regulators and how they work
/
[excuse the pause in the thread]
Medicine regulation was first introduced in the USA following deaths from unsafe treatments like Koremlu Cream and Elexir Sulfanilamide: really unsafe remedies for non-life-threatening conditions.
/
Medicine regulation was first introduced in the USA following deaths from unsafe treatments like Koremlu Cream and Elexir Sulfanilamide: really unsafe remedies for non-life-threatening conditions.
/

The FDA in the US refused to licence "the world's totally safe sleeping pill", Thalidiomid, as the FDA was not happy with the clinical trial design.
Europe lacked proper medicine regulation, Thalidomide was marketed, and many Europeans were born with horrible deformities.
/
Europe lacked proper medicine regulation, Thalidomide was marketed, and many Europeans were born with horrible deformities.
/

Thalidomid showed that medicines regulation works. Regulators have twin role: to protect patients from companies who are deceitful, incompetent or careless.
And to support innovation to enable new medicines to get to patients especially in areas of high medical need.
/
And to support innovation to enable new medicines to get to patients especially in areas of high medical need.
/
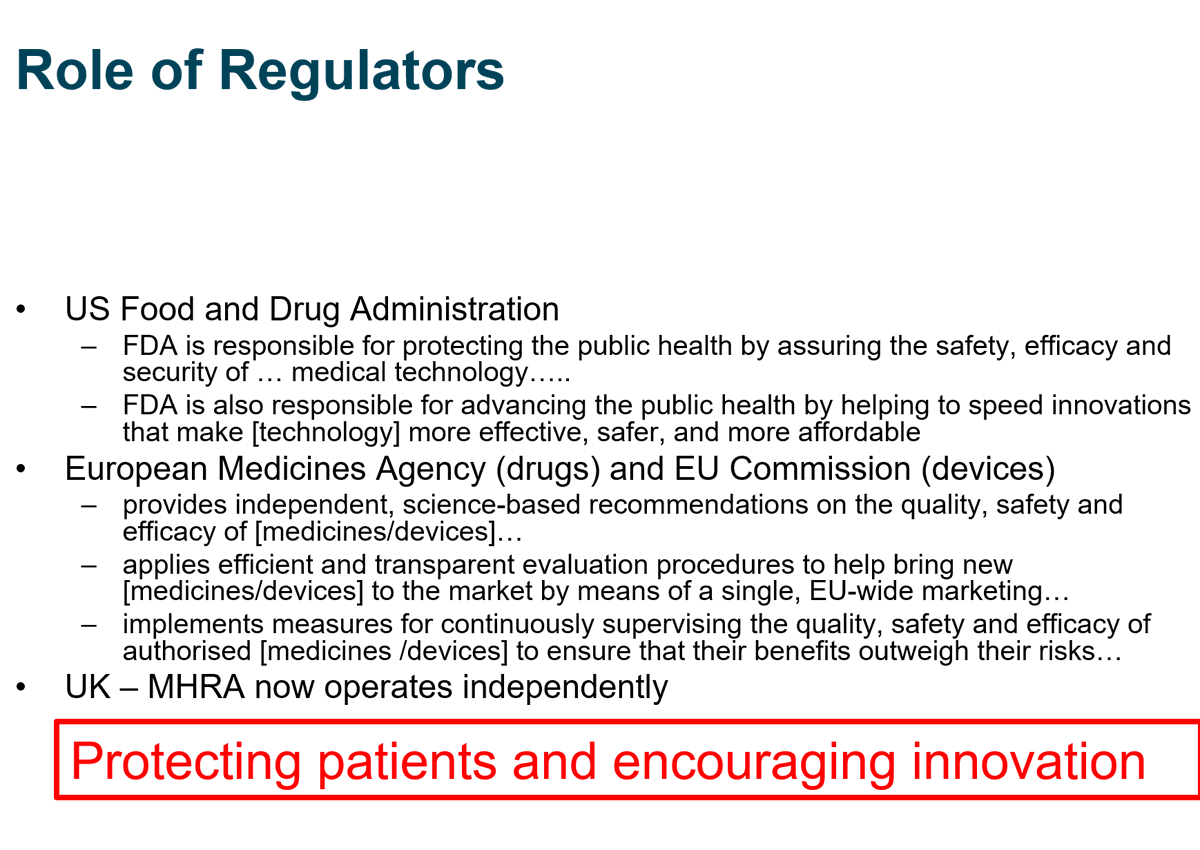
Regulators insist all development of medicines follows strict rules to ensure informed consent, proper design, meticulous records, careful analysis etc
They also issue issue specific guidance about requirements for specific treatments eg vaccines guidance from EMA, FDA
/


They also issue issue specific guidance about requirements for specific treatments eg vaccines guidance from EMA, FDA
/



These guidance documents are written after building consensus between many experts and regulators, and they go out to public consultation before being issued. It isn't just the opinion of a vocal doctor or company with vested interests. It takes account of everything we know.
/
/
Medicines regulation has strong foundations in risk assessment: what are the risk, how do we mitigate them, can we be confident the benefits outweigh the risks for a particular treatment and patient population?
/
/
You can't, therefore, compare a new medicines or vaccine to a product like asbestos, x-ray tubes, or cigarettes, introduced with no pre-market testing. It is precisely because we have lived with the consequences of drugs like Thalidomide that we have medicine regulation
/
/
If you say "but we can't trust the vaccines because they are too new", and "they might have side effects we won't know about for 50 years" you are basically saying medicines regulation cannot be trusted. Not just for vaccines, but for any innovative medicine.
/
/
Regulations are always evolving and improving. Regulators don't always make the right decision as they are humans. But to you accept the principle that we can develop innovative medicine for everything from life threatening cancer to erectile disfunction?
/
/
The argument that we can't trust new vaccines means we answer "no" to this question.
I would say the opposite: the evidence is that medicines regulation has served us well during the last few decades, and especially during this pandemic.
/
I would say the opposite: the evidence is that medicines regulation has served us well during the last few decades, and especially during this pandemic.
/
Medicines and vaccines do have side effects. Medicines regulation is about balancing risks so that benefits outweigh risks for the populations to be treated. More is learned once a drug is being used, and regulators review this post-market data and may refine their decision
/
/
As an individual you can reasonably say "this balance of risk and benefit doesn't feel right for me".
But to say "we can't trust vaccines because there might be unknow long term harm like cigarettes, x-rays, asbestos, is to say we we can't safely develop innovative vaccines
/
But to say "we can't trust vaccines because there might be unknow long term harm like cigarettes, x-rays, asbestos, is to say we we can't safely develop innovative vaccines
/
because
* no-one will do a 50 year clinical trial (even if it could be considered ethical)
* even if we could, any virus might have mutated before it finishes.
Is that really what you mean? If so, you are definitely anti Vax.
/
* no-one will do a 50 year clinical trial (even if it could be considered ethical)
* even if we could, any virus might have mutated before it finishes.
Is that really what you mean? If so, you are definitely anti Vax.
/
• • •
Missing some Tweet in this thread? You can try to
force a refresh