1. #VRBPAC is meeting today to review Novavax's submission for an EUA for its Covid vaccine. Often @matthewherper & I live blog VRBPAC meetings but not today. I'll try to live tweet.
Gonna be an interesting day.
If you want to watch the meeting: fda.gov/advisory-commi…
Gonna be an interesting day.
If you want to watch the meeting: fda.gov/advisory-commi…
2. #VRBPAC is going through roll call now. There's a big committee today — 23 (22 voting members, 1 non-voting). There's fully a dozen temporary members sitting on this panel. Tons of expertise here. A number of former VRBPAC members.
3. #VRBPAC is being asked whether Novavax's #Covid vaccine should be given an EUA. This is for their primary series (2 doses, given 3 weeks apart); they aren't asking for an EUA for use of their vaccine as a booster. It's based on the Wuhan (aka original) strain. 

4. Novavax's manufacturing problems are going to be part of today's #VRBPAC. Heard this right off the top with a statement I've never heard before: FDA effectively could not compare the vaccine used in the trials to the vaccine the company plans to market. 

5. This could be a rocky day for Novavax. Right off the top #VRBPAC member Eric Rubin asked how Novavax qualifies for an EUA, given the fact there are other vaccines available. @US_FDA's Peter Marks says that the statute allows leeway for a vaccine that meets an unmet need.
6. In the case of the Novavax vaccine, @US_FDA's Marks says that currently there aren't non-mRNA vaccines considered as first line products in the US and Novavax would fill that void. This might persuade some unvaccinated people to get vaccinated, he said.
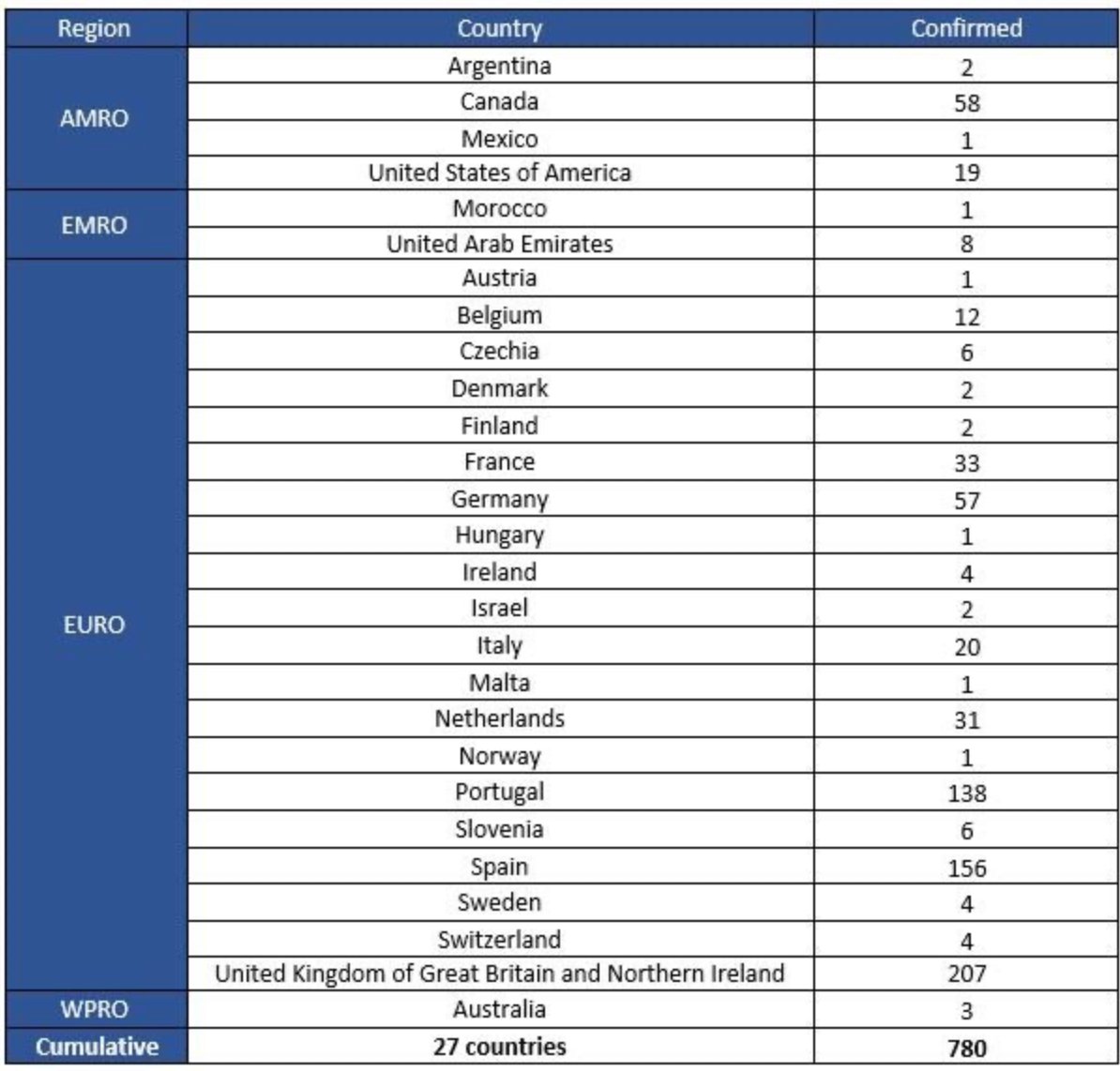
7. A presentation from Heather Scobie from @CDCgov shows that 27M Americans haven't had any vaccine doses yet. (But lots more aren't fully vaxed & boosted.) This slide shows who isn't vaxed by age, race & ethnicity. 

7. I haven't covered a #VRBPAC for a while. The technical challenges haven't changed in that time.
Alas.
Alas.
8. @CDCgov's Tom Shimabukuro is giving a review of myocarditis, a side effect seen with the mRNA vaccines & with the Novavax vaccine. He notes vaccine associated myocarditis associated with Covid vaccination is relatively mild compared to reg. myocarditis.
statnews.com/2022/06/03/fda…
statnews.com/2022/06/03/fda…

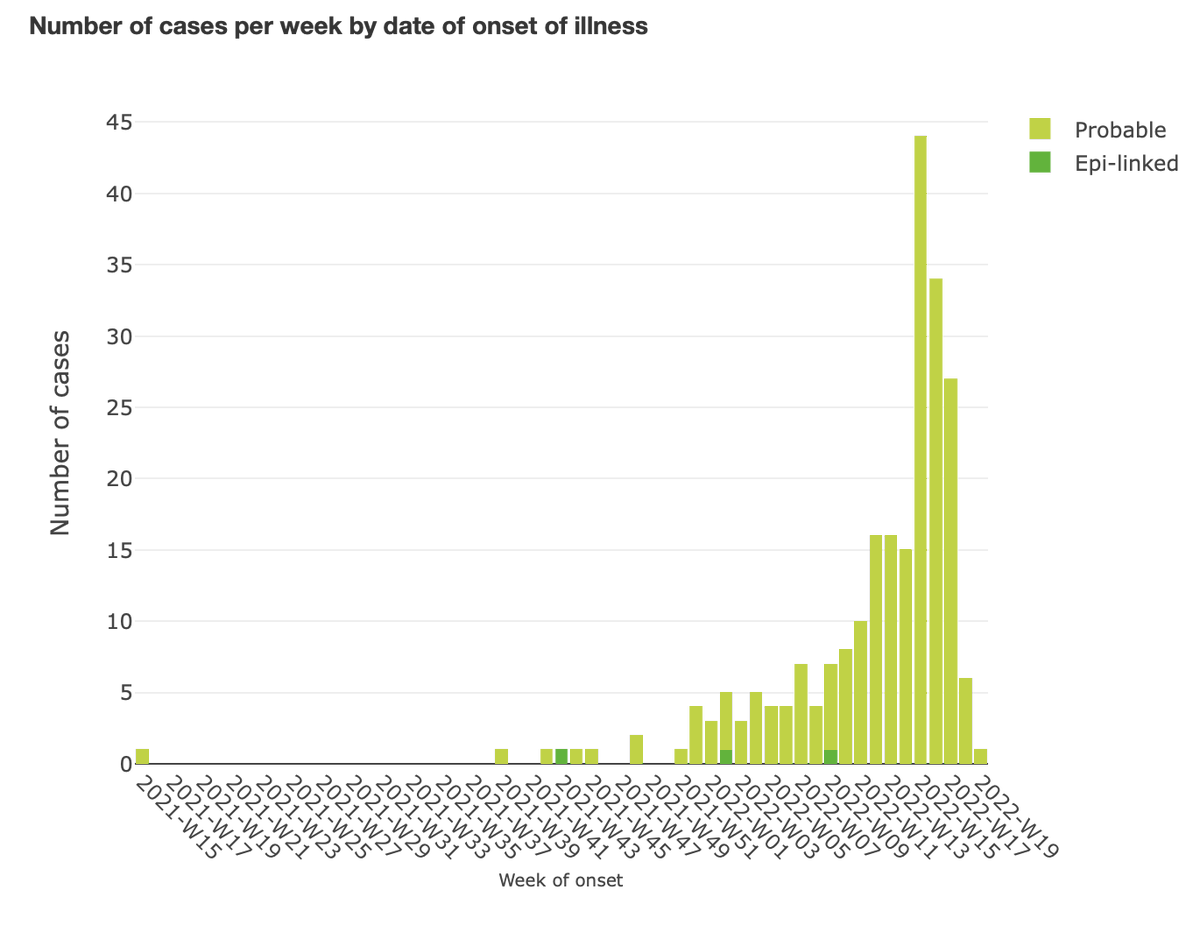
9. Shimabukuro is presenting a lot of data on myocarditis — data @CDCgov has been collecting since the start of the US Covid vaccination program. I'm not going to try to keep up with him. You can view his slides here: fda.gov/media/159007/d…
10. Hard to watch #VRBPAC - the sound and the video isn't in sync. I can see 2 people speaking at once, but hearing only 1 voice.
11. Novavax's press office reached out with comment on FDA's comments in Tweet #4 in this thread.
I'm not sure how this clears up the situation, but this is what they said.
I'm not sure how this clears up the situation, but this is what they said.

13. Denny Kim, Novavax's chief safety officer, reports that they saw an imbalance of cholecystitis (inflammation of the gall bladder) in the vaccine arms of their trial. Analysis doesn't support a causal link, he said. 

14. Kim is picking at the myocarditis & pericarditis data. He said the totality of evidence does not support a causal link.
In post-authorization use of the vaccine in countries where it has been approved, only Australia, which used 17% of 744,000 doses, has reported myocarditis
In post-authorization use of the vaccine in countries where it has been approved, only Australia, which used 17% of 744,000 doses, has reported myocarditis

15. Kim says there were no reports of anaphylaxis (a problem seen with mRNA vaccines) and no cases of TTS (seen with the J&J and AZ vaccines). There was 1 case of GBS. 

16. @US_FDA is now presenting on their analysis of the Novavax data. The slide deck that corresponds to this presentation is here: fda.gov/media/159004/d…
17. "The conference call is now ending" is not what you expect to hear 5 hours before a #VRBPAC meeting is scheduled to end. @US_FDA
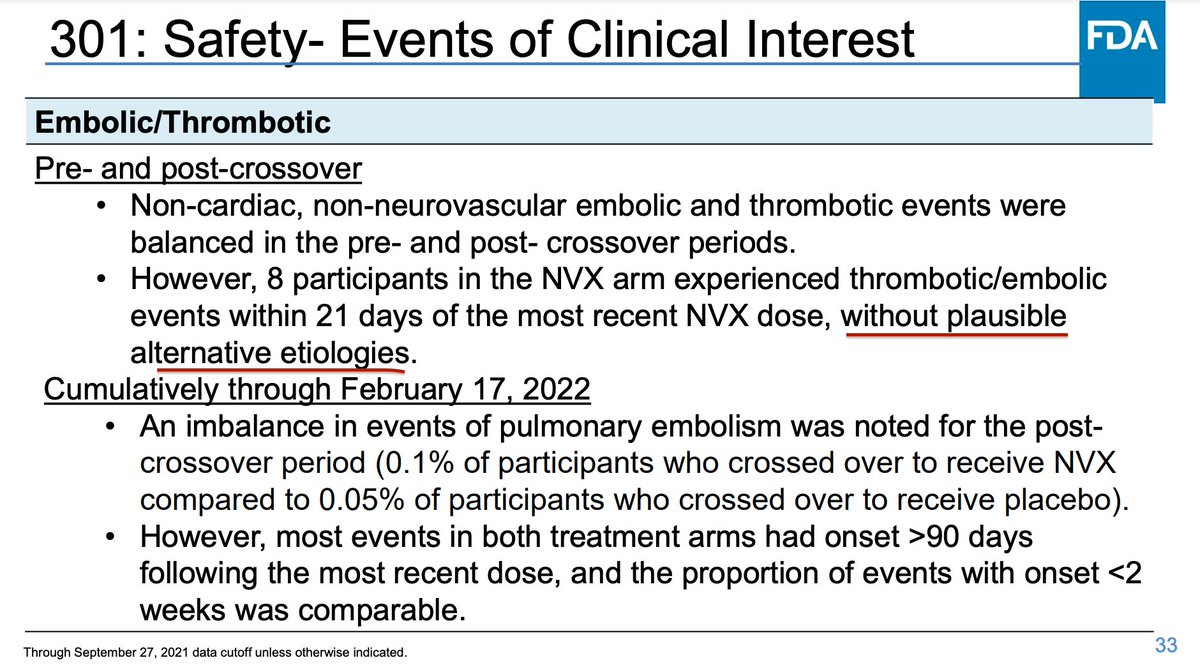
19. The @US_FDAm analyst, Lucia Lee, is presenting data on adverse events in clinical trials Novavax ran. There was an imbalance of events consistent with stroke in the 15 days after a dose of vaccine, she said. 

20. Lee said there was also an imbalance in embolic and thrombotic events in the vaccine arms within 21 days of a dose "without plausible alternative etiologies." 
21. Lee mentioned as concerning 5 cases of myocarditis which occurred within 20 days of vaccination. 

22. #VRBPAC breaks for lunch after surprisingly few questions. Public comment portion of the meeting runs from 1 pm-2 pm.
If you're interested in the Novavax vaccine, here's some lunch break reading: @Dereklowe's column today.
science.org/content/blog-p…
If you're interested in the Novavax vaccine, here's some lunch break reading: @Dereklowe's column today.
science.org/content/blog-p…
23. The public hearing portion of #VRBPAC underway now.
Speakers are asked to state if they have any conflicts of interest, but aren't required to.
I personally would like to know if speakers own stocks in the company whose application they are weighing in on.
Speakers are asked to state if they have any conflicts of interest, but aren't required to.
I personally would like to know if speakers own stocks in the company whose application they are weighing in on.
24. Back to the main show at #VRBPAC. Novavax is being given a chance to provide additional information "given that all the testing was done in the era of Alpha and we're getting ready to launch the vaccine in the era of Omicron." — acting chair Arnold Monto.
25. Novavax chief medical officer Filip Dubovsky acknowledged the company doesn't have efficacy data against Omicron, but immunogenicity data looks good and Novavax believes features of its vaccine engender broad cross protection. #VRBPAC.
26. Sounds like the technician running this show often isn't muted. #VRBPAC
27. Chair Arnold Monto asked Novavax to explain why there are differences between the vaccine in its clinical trial & the vaccine it is seeking to license. Dubrovsky, chief med officer, explained that the company has generated comparability data on the different lots of vaxes. 

28. But Dubrovsky said the company acknowledges "that FDA has a perspective on this that is different from ours."
The vaccine being used in a number of countries & which would be used in the US — if the vaccine is authorized — was made at Serum Institute of India.
The vaccine being used in a number of countries & which would be used in the US — if the vaccine is authorized — was made at Serum Institute of India.
29. #VRBPAC member Art Reingold asked about co-administration of the Novavax vaccine with flu vaccine. Dubrovsky said one of their studies showed coadministration led to a depression of the Covid response, but that could be fixed by dialing down the flu HA & up the Covid vax.
27. #VRBPAC begins the discussion of the voting question.
Eric Rubin notes that the Novavax data look very similar to those generated by the mRNA vaccines. But he expressed disappointment that there aren't more recent data. Mark Sawyer echoed that disappointment.
Eric Rubin notes that the Novavax data look very similar to those generated by the mRNA vaccines. But he expressed disappointment that there aren't more recent data. Mark Sawyer echoed that disappointment.
28. They both would have liked to see how well this vaccine would have held up against Omicron. The data Novavax is presenting are from a trial that ended last fall. But both said their understanding of #VRBPAC's job is to assess the data they've seen & leave the decision to FDA.
29. This is the question #VRBPAC is going to vote on. Bruce Gellin, who is temporary member for this meeting, notes that Novavax has more data than is being considered today. 

30. @US_FDA's Doran Fink says FDA believes there are important differences in lots of Novavax vaccine used in different trials that the company ran. So some trial results were not included in the final package. "What you're seeing is the product you're getting" said Peter Marks.
31. #VRBPAC members are discussing whether @US_FDA should require a label warning of myocarditis. Novavax continues to push back, saying they don't believe there is sufficient evidence to assign a causal relationship.
32. @US_FDA's Doran Fink said a warning can be required if there is "reasonable evidence of a causal association."
No #VRBPAC member argued that there should NOT be a warning about myocarditis on the label.
No #VRBPAC member argued that there should NOT be a warning about myocarditis on the label.
33. #VRBPAC member Ofer Levy asked if @US_FDA is going to release a pecking order of #Covid vaccines so that people can understand which vaccines the agency thinks is best. (FDA recently recommended the J&J vaccine be considered a 2nd line vaccine.)
34. CBER director Peter Marks said @US_FDA is looking at making another vaccine available. He said @CDCgov (through #ACIP) might do this.
FDA's Doran Fink said the J&J decision was based on the serious safety concern, TTS, which is associated with it.
FDA's Doran Fink said the J&J decision was based on the serious safety concern, TTS, which is associated with it.
35. #VRBPAC is going to wrap up early today. Voting is about to start.
Prediction: This vaccine will be approved.
Prediction: This vaccine will be approved.
36. #VRBPAC has voted to recommend that @US_FDA authorize the Novavax Covid vaccine by a vote of 21 yeses, 0 noes, 1 abstention.
FDA doesn't have to follow VRBPAC's advice but it generally does.
FDA doesn't have to follow VRBPAC's advice but it generally does.

37. The one abstention was temporary member Bruce Gellin, former head of HHS's national vaccine program office and now chief of global public health strategy at the Rockefeller Foundation.
Gellin says he would have liked to vote a provisional yes, but that wasn't an option.
Gellin says he would have liked to vote a provisional yes, but that wasn't an option.
38. Gellin said the data Novavax presented showed it was safe and worked back when the studies were conducted. But it's not known whether that continues today.
Said it will be important to evaluate the product going forward if it's authorized.
Said it will be important to evaluate the product going forward if it's authorized.
39. Gellin and several of the #VRBPAC members credited Novavax for persevering with their vaccine development, despite a lot of problems in their route to today.
Meeting concluding.
Meeting concluding.
• • •
Missing some Tweet in this thread? You can try to
force a refresh