The final version of our study seeking to understand how even mild respiratory COVID can affect the brain is now published. Sharing an updated thread… 1/
Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation cell.com/cell/fulltext/…
Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation cell.com/cell/fulltext/…
COVID survivors frequently suffer from lasting cognitive symptoms. Noting the clinical similarities between “chemo-fog” and “COVID-fog”, we asked whether there are also neurobiological similarities, in collaboration with Akiko Iwasaki’s @VirusesImmunity lab @YaleMed. 2/
How might even mild respiratory #COVID infection affect the brain? Using a mouse model of mild SARS-CoV-2 infection limited to the respiratory system, we found elevation of CNS cytokines/chemokines and white matter-specific microglial reactivity. 3/ 

We found the same histological pattern of white matter-selective microglial reactivity in the brains of people with #COVID that we found after chemotherapy (tinyurl.com/Cell-2019), in collaboration with @nathavindra, #MyounghwaLee #RebeccaFolkerth #DanPerl #MarcoHefti 4/ 

Single cell RNA sequencing of microglia in mice after mild respiratory COVID revealed a state of reactivity that shares features with “disease-associated microglia" in various contexts, but with COVID-specific features as well. With @michaelrodea and @LiddelowLab 5/ 

One of the #chemokines elevated is CCL11, known to be associated with cognitive impairment. We found elevated CCL11 levels in people suffering from #LongCOVID with cognitive symptoms compared to those with no cognitive symptoms. Collaboration with @PutrinoLab @MountSinaiNYC 6/ 

Building on foundational work by @VilledaLab, we tested direct effects of systemic CCL11 administration and found that CCL11 causes microglial reactivity: but ONLY in the hippocampus. 7/ 

(The region/circuit-specific effect of a chemokine was unexpected, and raises the possibility that various immune challenges, eliciting different cytokine/chemokine profiles, may increase risk for overlapping yet distinct constellations of neurological & psychiatric symptoms) 8/
Like the effects of microglial reactivity after cancer therapy (tinyurl.com/Science-2003), we found impairment in hippocampal neurogenesis. CCL11 was sufficient to cause this loss of new hippocampal neurons. 9/ 

Also like the effects of cancer therapy (tinyurl.com/Cell-2019), we found a loss of myelinating oligodendrocytes after mild respiratory COVID in subcortical white matter. Comparing mouse models, we found a similar loss of myelin after chemo or COVID. 10/ 
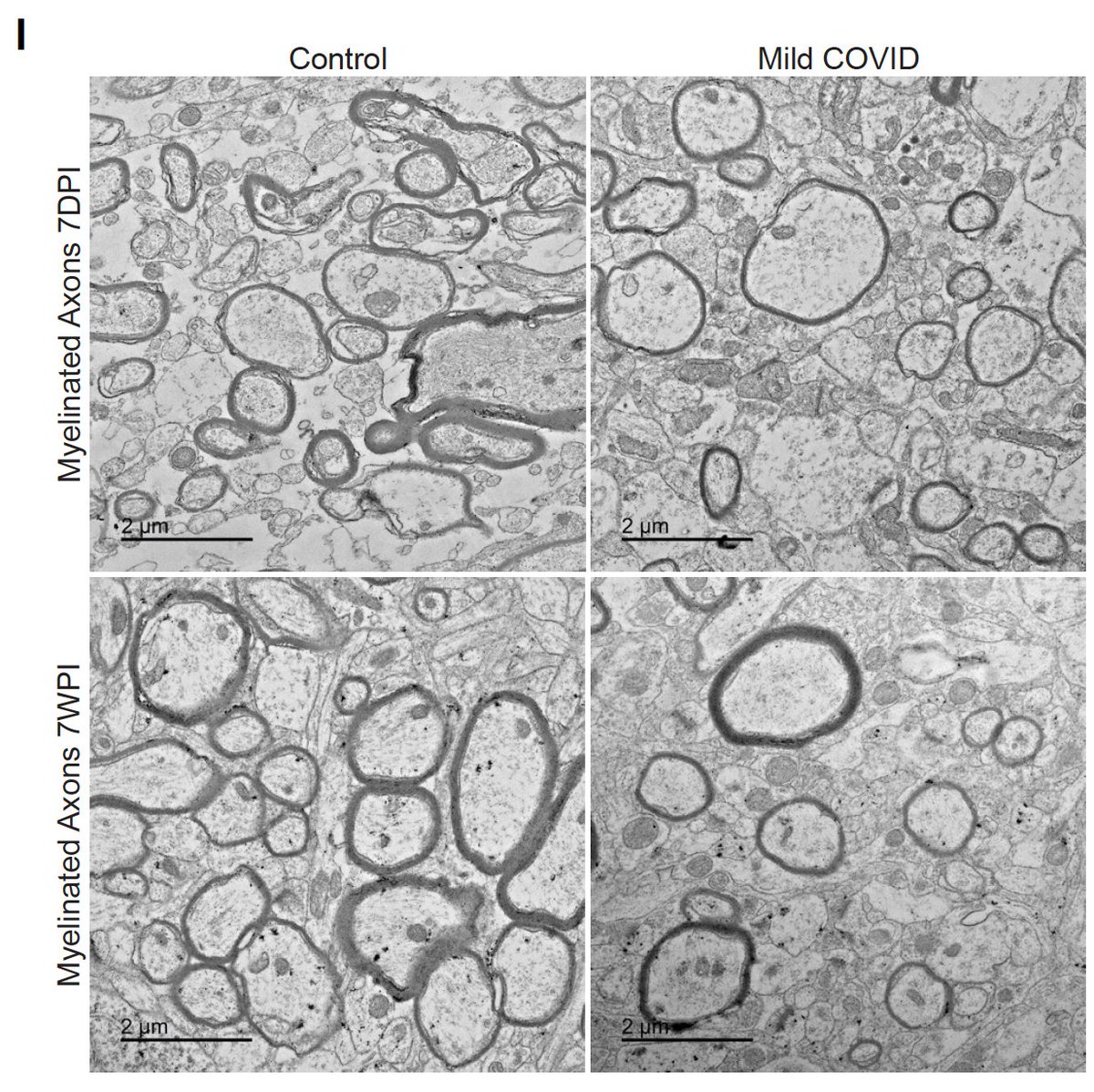
Mild respiratory influenza A caused similar neuroinflammation and cellular dysregulation in mice at one week post-infection, but many of these changes were not as long-lasting as after COVID, with normalization of white matter changes by 7 weeks after influenza. 11/ 

One shared finding in both COVID and influenza models of respiratory infection was persistent elevation in CCL11 and lasting decrease in hippocampal neurogenesis 12/
Notably after #influenza, additional cytokines became elevated in the CSF of mice by 7 weeks after infection. This is concordant with the prominent neurological and neuropsychiatric sequelae reported in past influenza pandemics, and requires further study. 13/
Together, the findings in these mouse models of mild respiratory COVID and influenza highlight the principle that the immune response to infections outside of the brain can cause neuroinflammation and consequently neural cell dysregulation that can affect brain function. 14/ 

There are stark parallels between the cellular dysregulation that can happen after chemotherapy and even mild #COVID. Understanding the neurobiological underpinnings of #LongCOVID will lay the groundwork for therapeutic strategies to address this neurological health crisis. 15/
Deeply grateful for this wonderfully collaborative team effort, led by first authors @ThisIsAnthonyFC @AnnaGeraghty2 @peowenlu @ericsongg, and for support from @HHMINEWS, @NIH_CommonFund @NINDSnews @NIAIDNews and many others. 16/16
And now, the open-access, copy-edited version:doi.org/10.1016/j.cell…
• • •
Missing some Tweet in this thread? You can try to
force a refresh








