1/19 Welcome to this #CME #Tweetorial on #Influenza and #PediatricFlu with @RishiDesaiMD, supported by an educational grant from Genentech.
See full CME info: bit.ly/3Eczmx6
#MedTwitter #MedEd
See full CME info: bit.ly/3Eczmx6
#MedTwitter #MedEd

2/Let’s begin!
Influenza (flu) can cause severe illness and death. In the US, during the 2019-2020 (pre-pandemic) flu season, 20,000 deaths occurred due to flu-related complications.
Influenza (flu) can cause severe illness and death. In the US, during the 2019-2020 (pre-pandemic) flu season, 20,000 deaths occurred due to flu-related complications.

3/During the pandemic, social distancing, masking, and quarantining significantly reduced flu-related illnesses/deaths. In the US, during the 2021-2022 (pandemic) flu season, 5,000-14,000 deaths occurred due to flu-related complications (down from 20,000!). 



4/Vaccines are one way to reduce flu transmission and disease burden. In a “well-matched” season, vaccines reduce the risk of illness by 40%-60%, but in a “mismatched” season (when the dominant flu strain is different from the one in the vaccine), vaccines are less effective.
5/Time for a poll! What percentage of children aged 6 months to 4 years received the flu vaccine in 2020-2021?
6/If you answered B (68%), you are correct! The percentage of children who got the flu vaccine was highest among those aged 6 months to 4 years (68%), followed by children 5-12 years (59%), and lowest among children 13-17 years (51%). 

7/In the ongoing pandemic, it’s important to keep in mind that flu symptoms overlap with COVID-19 symptoms, and the two viruses can co-occur. 
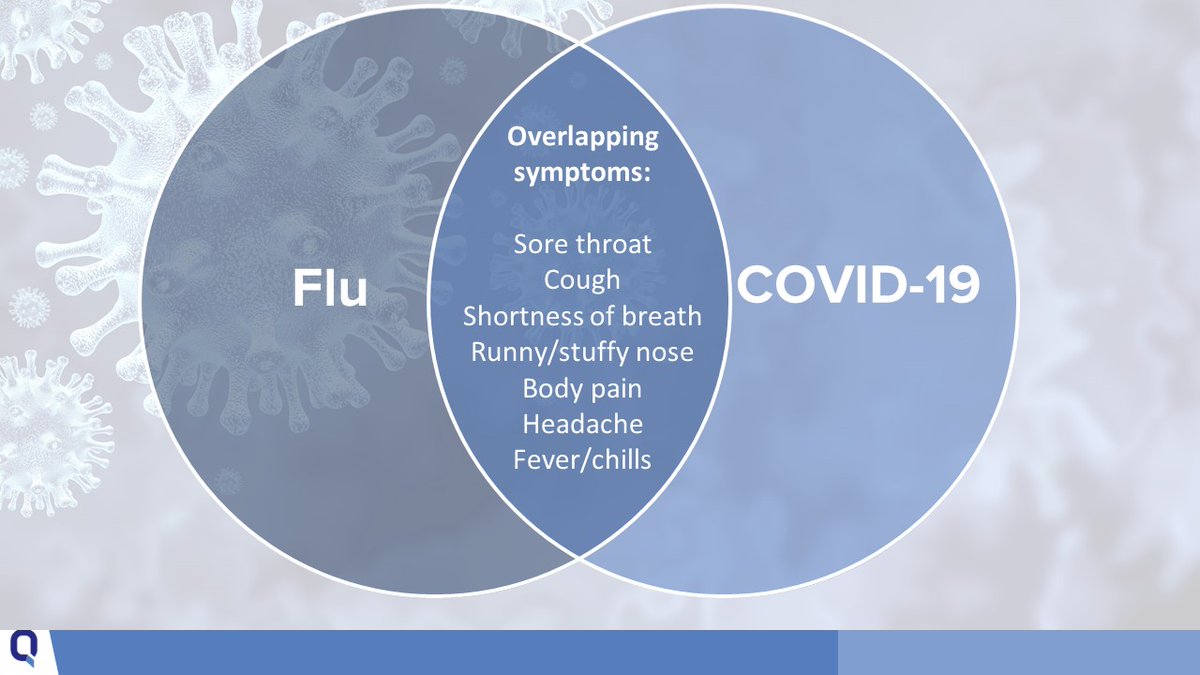
8/Testing can help determine whether symptoms are caused by flu or COVID-19. Flu tests have limitations, for example:
• Rapid antigen tests have a high false-negative rate
• Viral cultures take 3-10 days for results
• RT-PCR can be expensive
• Rapid antigen tests have a high false-negative rate
• Viral cultures take 3-10 days for results
• RT-PCR can be expensive

9/The #CDC recommends starting antiviral treatment as soon as possible (≤ 48 hours) for patients with confirmed/suspected influenza who have or are at risk of severe disease, such as young children.
10/M2 proton channel blockers (amantadine and rimantadine) have been used historically as antiviral treatment but have high rates of resistance and aren’t recommended in the US.
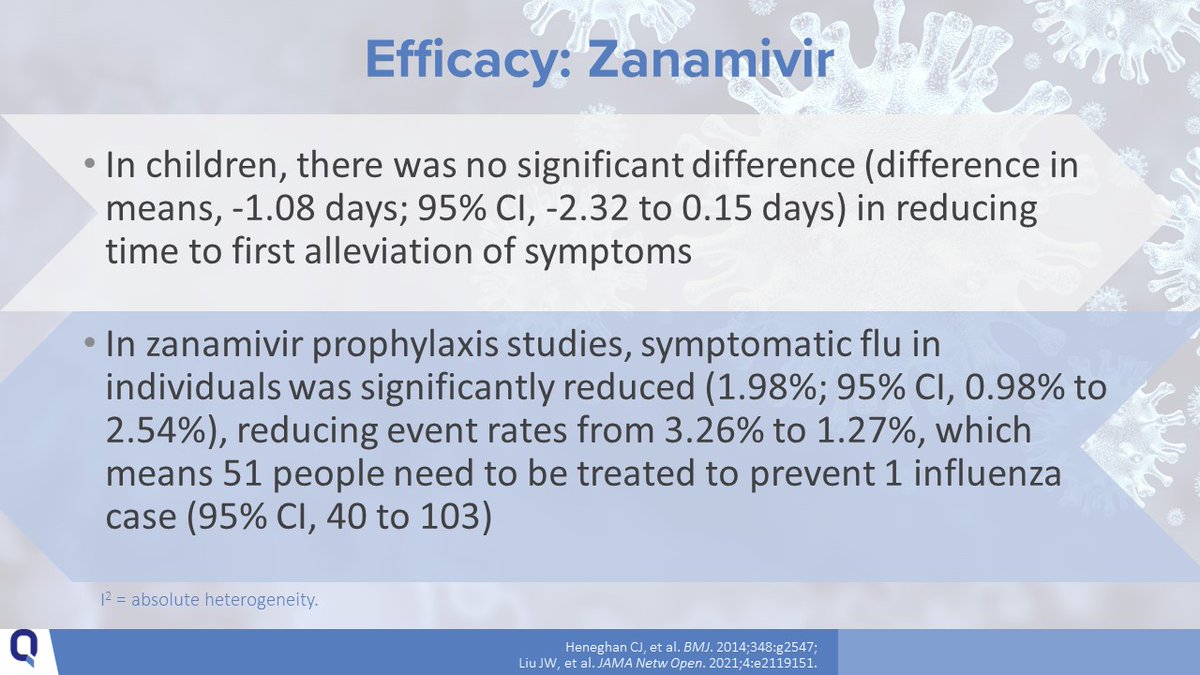
12/Zanamivir (INH BID x 5 days) can be used in children aged ≥ 7 years but is not recommended in people with underlying respiratory disease. 
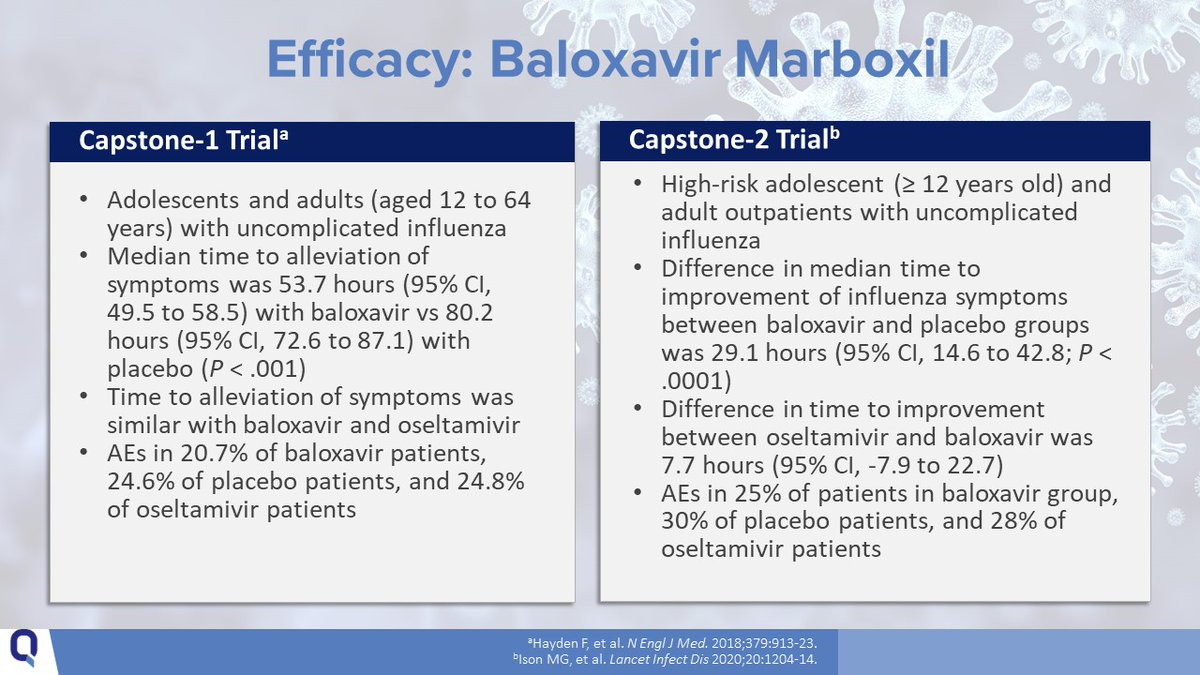
14/Baloxavir Marboxil (aka baloxavir) (1 PO dose) can be used in children aged 5-12 years with no chronic medical condition and in any child aged ≥ 12 years. 


16/For outpatients with suspected or confirmed uncomplicated influenza, oral oseltamivir, INH zanamivir, IV peramivir, or oral baloxavir may all be considered for treatment. Baloxavir has been shown to be more effective than oseltamivir against influenza B. 

17/Time for another poll! Which of the following would be appropriate for treating uncomplicated influenza in an otherwise healthy 6-year-old boy?
I.Baloxavir (1 PO dose)
II.Oseltamivir (PO BID x 5 days)
III.Zanamivir (INH BID x 5 days)
IV.Peramivir (1 IV infusion)
I.Baloxavir (1 PO dose)
II.Oseltamivir (PO BID x 5 days)
III.Zanamivir (INH BID x 5 days)
IV.Peramivir (1 IV infusion)
18/The correct answer is D: uncomplicated influenza in an otherwise healthy 6-year-old boy can be treated with baloxavir (1 PO dose), oseltamivir (PO BID x 5 days), or peramivir (1 IV infusion). Zanamivir is not indicated in children younger than 7 years.
19/Thank you for joining this #CME #Tweetorial!
To earn CME credit or view references & glossary: bit.ly/3Eczmx6
To earn CME credit or view references & glossary: bit.ly/3Eczmx6
@threadreaderapp unroll please
• • •
Missing some Tweet in this thread? You can try to
force a refresh


















