
🧵#ScienceBreakdown: "Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors"
Interesting new paper by @DEOlsonLab, @LinTianPhD, et al. looking at why some serotonin 2A receptor agonists promote neuroplasticity, but others do not.
1/
Interesting new paper by @DEOlsonLab, @LinTianPhD, et al. looking at why some serotonin 2A receptor agonists promote neuroplasticity, but others do not.
1/

Various small molecules, from endogenous neurotransmitters like serotonin to tryptamine #psychedelics, activate 5HT2A receptors... and yet they can lead to very different effects.
Getting at why this is was one of the basic motivations for this study.
2/
Getting at why this is was one of the basic motivations for this study.
2/

One idea here is that various compounds have distinct physical/chemical properties, despite all activating 5HT2A receptors.
For example, they differ in fat solubility. Some can cross cell membranes to get *inside* cells, and some can't...
3/
For example, they differ in fat solubility. Some can cross cell membranes to get *inside* cells, and some can't...
3/

In cultured neurons, they measured compounds' ability to induce plasticity. Bathe cells in cmpds, then assessed plasticity by counting # of "spines" (places where synapses are) or dendritic complexity (# of times dendrites cross each other).
Did so for multiple cmpds:
4/
Did so for multiple cmpds:
4/
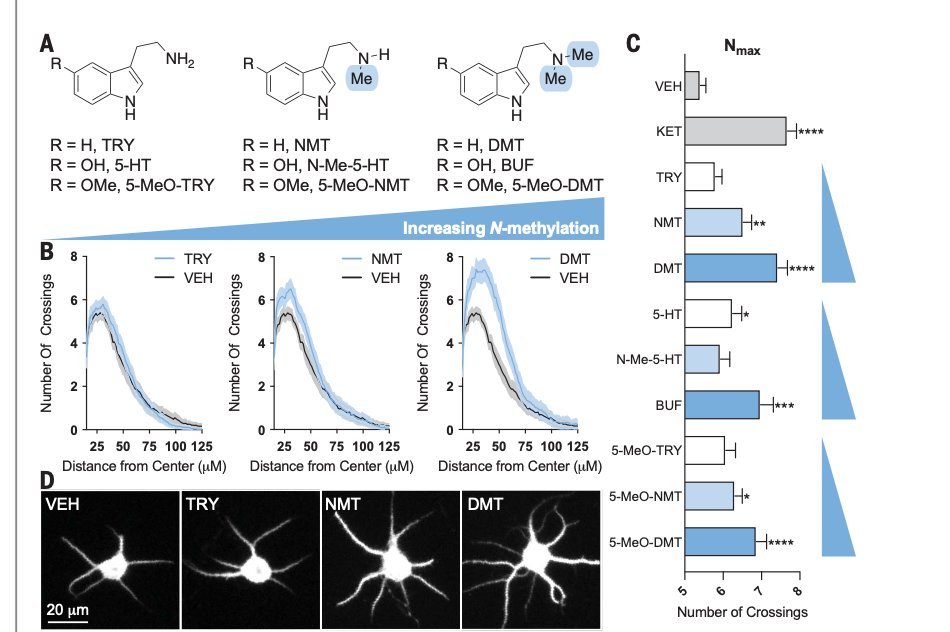
Graphs show that some cmpds induce more structural plasticity than others. This is tied to chemical properties.
Cmpds with more "N-methylation" induce more plasticity. This is includes #psychedelics like 5-MeO-DMT, which induces more plasticity than serotonin or tryptamine.
5/

Cmpds with more "N-methylation" induce more plasticity. This is includes #psychedelics like 5-MeO-DMT, which induces more plasticity than serotonin or tryptamine.
5/


Basically, the more fat soluble a 5HT2A receptor agonist is, the more plasticity you see.
(there's some more complicated stuff going on here related to how cmpds different molecular signaling pathways, which I'm skipping over).
6/

(there's some more complicated stuff going on here related to how cmpds different molecular signaling pathways, which I'm skipping over).
6/


"Psychoplastogen" is a new term referring to compounds, like classic #psychedelics, which can rapidly induce neuroplasticity.
For background on this, see my convo w/ @DEOlsonLab: mindandmatter.substack.com/p/podcast-46-d…
For criticism, see my convo w/ Gul Dolen: mindandmatter.substack.com/p/gul-dolen-so…
7/
For background on this, see my convo w/ @DEOlsonLab: mindandmatter.substack.com/p/podcast-46-d…
For criticism, see my convo w/ Gul Dolen: mindandmatter.substack.com/p/gul-dolen-so…
7/
So, more fat soluble cmpds seem to be more plasticity-inducing. Idea is that perhaps this is b/c they're actually going inside cells to activate 5HT2A receptors, rather than doing so via receptors on the outer cell membrane (which is what we normally think of drugs doing).
8/
8/

5HT2A receptors are GPCRs (a general type of receptor). Most of the time, you find, GPCRs on outer cell membranes, but there are some known to be localized inside of cells.
Next step is to see where 5HT2A receptors are localized in these neurons. Outside, inside, or both?
9/
Next step is to see where 5HT2A receptors are localized in these neurons. Outside, inside, or both?
9/
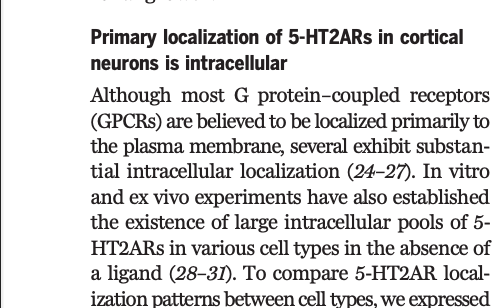
Figure is kind of tough to explain, but they find evidence for 5HT2A receptors localized inside of neurons.
That does not mean 5HT2A receptors are *only* on the inside of neurons, but a sizable pool of these receptors appears to be inside of the neurons they looked at.
10/
That does not mean 5HT2A receptors are *only* on the inside of neurons, but a sizable pool of these receptors appears to be inside of the neurons they looked at.
10/

Must these cmpds actually go inside of cells to have their plasticity-inducing effect? To test this, they chemically modified DMT, psilocin, etc. so they had reduced membrane permeability, but could still activate 5HT2A receptors.
11/
11/

When DMT & psilocin modified to be less fat-soluble, didn't induce as much plasticity unless "electroporation" was used to force them inside cells.
Indicates that plasticity from cmpds like DMT & psilocin largely due to crossing cell membrane & activating 5HT2A inside cells
12/
Indicates that plasticity from cmpds like DMT & psilocin largely due to crossing cell membrane & activating 5HT2A inside cells
12/

What about the opposite? Can a cmpd like serotonin, which can't naturally cross membrane, be somehow forced into neurons? And if so, will it then induce plasticity similar to more fat soluble #psychedelics like DMT & psilocin?
13/
13/

Skipping details, the answer appears to be, "Yes."
If you force serotonin into cells, it now induces structural plasticity more than it otherwise would.
14/
If you force serotonin into cells, it now induces structural plasticity more than it otherwise would.
14/

Effects of #psychedelics on neuroplasticity are related to their observed antidepressant effects.
Since forcing serotonin inside of cells made it "look like" #psychedelics in terms of plasticity effect, can this also enable it to induce antidepressant effects similar to psychs?
Since forcing serotonin inside of cells made it "look like" #psychedelics in terms of plasticity effect, can this also enable it to induce antidepressant effects similar to psychs?
Short answer appears to be, "Yes."
That's all I have time to unpack here, but this is a really cool study worth checking out if you're interested in the details of how #psychedelics work in the brain at the cellular/molecular level to induce their effects.
16/16
That's all I have time to unpack here, but this is a really cool study worth checking out if you're interested in the details of how #psychedelics work in the brain at the cellular/molecular level to induce their effects.
16/16

• • •
Missing some Tweet in this thread? You can try to
force a refresh











