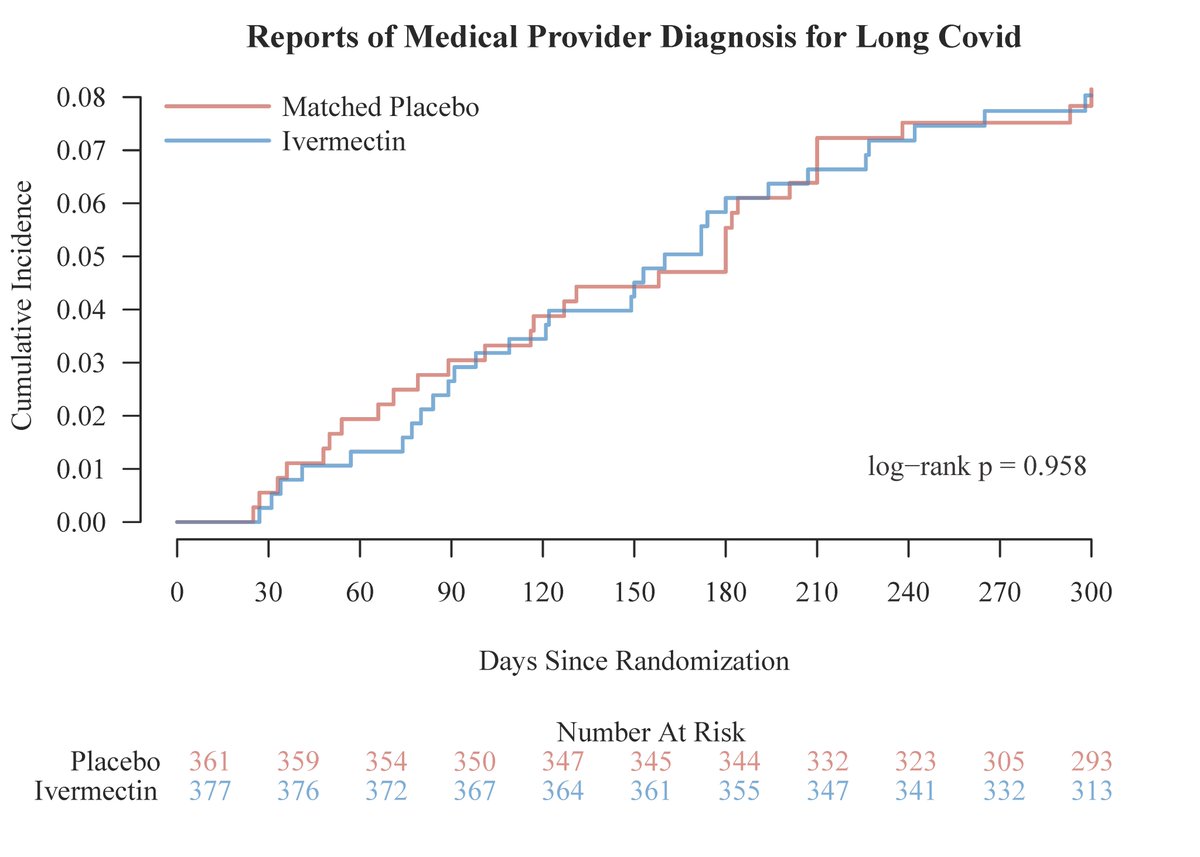
Ivermectin still does not work when dosed up to 6 days at doses off 400-600 mcg/kg/day for #covid19
Read the manuscript.
We're presenting virologic data tomorrow at #CROI from Covid-OUT trial on metformin, IVM, & fluvoxamine. One is very interesting.
jamanetwork.com/journals/jama/…
Read the manuscript.
We're presenting virologic data tomorrow at #CROI from Covid-OUT trial on metformin, IVM, & fluvoxamine. One is very interesting.
jamanetwork.com/journals/jama/…
Editorial by the JAMA Editor in Chief which summarizes the situation.
jamanetwork.com/journals/jama/…
jamanetwork.com/journals/jama/…
Let's talk about time of enrollment which reflects real world reality of people seeking testing and then seeking care. With the trial there is an inherent delay to consent, enroll, and ship drug. 44% of participants started within 4 days of symptom onset. 

Best figure in #ivermectin manuscript (actually the Appendix) is eFigure4, which I pushed for inclusion. Regardless of how early one started IVM after #covid19 symptom onset, no benefit was observed regarding duration of symptons, including with those with severe symptoms. 

Time to start therapy could be very important if #ivermectin actually had anti-viral properties in humans. It does not.
The Covid-Out trial looked at viral load change over time
More on that later today.
The Covid-Out trial looked at viral load change over time
More on that later today.
Covid_Out trial data presented at CROI today on the lack of virologic effect for #ivermectin when compared to an identical matched placebo control. There is not an antiviral effect when dosed at 400 mcg/kg/day for 3 days. 

Medications can have immunomodulatory effects or other potential mechanisms for clinical benefit which could be not directly or indirectly antiviral by mechanism. But -- in neither the TogetherTrial or Covid-Out was any antiviral effect observed with IVM in humans.
• • •
Missing some Tweet in this thread? You can try to
force a refresh