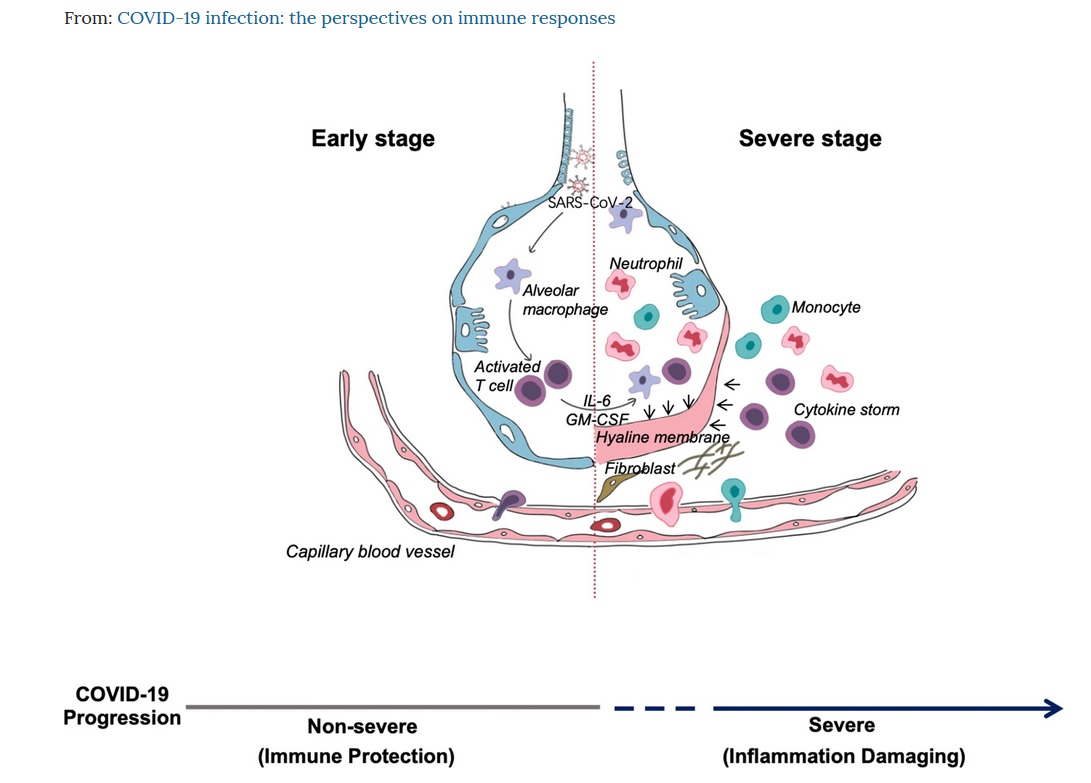
In this thread, I’ll speak about #tocilizumab and challenges to access it. Tocilizumab is an anti-IL-6-receptor monoclonal antibody. It is a promising investigational drug to treat the cytokine storm associated with severe #COVID19.
Tocilizumab is not an antiviral agent. Like other host focused therapies for COVID19, tocilizumab can be used in association with an effective antiviral and their combined actions may be complementary.
Tocilizumab is only for severe COVID19 cases who will need other forms of supportive care. It is administered IV (or SC) and requires strict supervision.
In addition to tocilizumab, other anti-IL6/IL6R mAbs are sarilumab, clazakizumab and siltuximab.
Other potential drugs to treat the cytokine storm include JAK inhibitors (i.e. baricitinib, ruxolitinib), which are small oral molecules (not mAbs).
Keep an eye on them too.
Tocilizumab is approved in many countries for rheumatoid arthritis. Originator product names are Actemra and RoActemra by @Roche/@Genentech. Also approved for CAR-T-related cytokine release syndrome. Hence the idea to use it against cytokine storm during COVID-19.
Following this publication, some frontline doctors in Naples, Italy called their colleagues in China, reviewed the evidence and started off-label use of tocilizumab in severe #COVID19 patients too.
ilriformista.it/buonaguro-il-v…
Since then, several small case series have reported encouraging outcomes for tocilizumab. The latest report however noted that a higher dose may be needed in critical patients.
Clinical trials of tocilizumab are on-going in Italy, US and France, among others. Results of the RCT in Italy are expected within a few weeks.
metaevidence.org/viewPathology2…
Tocilizumab is an immunosuppressant, thus patients may be at increased risk of infections following administration. This video tells you all about the risks and side effects. Particular attention should be paid to possible activation of latent TB.
Now let’s speak about access to tocilizumab. Tocilizumab is currently in short supply. I have been saddened to read many tweets literally begging for help… Just one example here…
The short supply of tocilizumab at global level has been worsened by the purchase of 10,000 vials in March by the US Strategic National Stockpile. Another example of hoarding of medicines…
The tocilizumab market is extremely profitable for @Roche. Sales of Actemra reached 2.2 billion USD (or CHF) in 2018.
AFAIA retail price of one 400mg vial in LMICs seems to be between ~400 USD (China) & ~800 USD (Morocco).
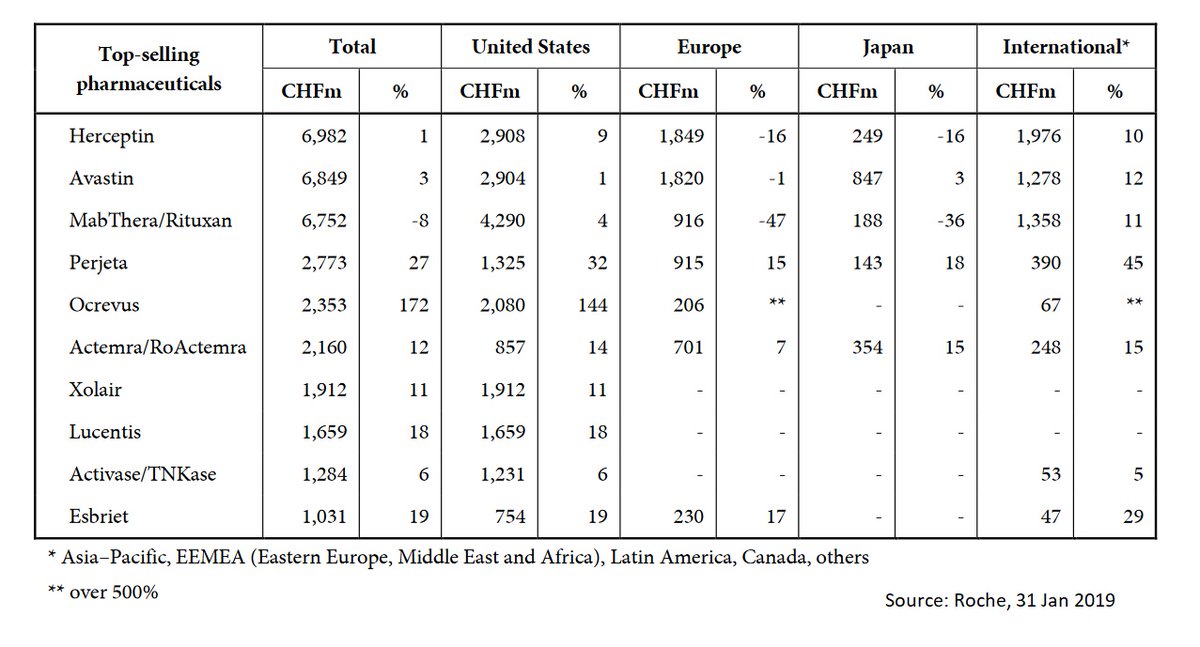
Good news: tocilizumab is now off-patent (since 2017). However, no approved biosimilar yet.
Approval of biosimilars is known to be a complicated process, requiring costly comparative clinical trials between originator product and biosimilar…
healthpolicy-watch.org/access-to-affo…
There are several tocilizumab biosimilar candidates in the pipeline, at different stages.
Examples: AryoGen (Iran), Bio-Thera (China), Hisun (China), Mycenax (Taiwan), Serum Institute (India), @Bioconlimited (India), @FreseniusKabi (Switzerland), @EpirusBiopharma (USA).
Let’s assume a moment that tocilizumab is found effective for COVID-19. Given urgency and shortage, maybe regulators, following a B/R analysis, will work to expedite approval of tocilizumab biosimilars without requiring a comparative clinical trial w/ Actemra...🙏
@Roche/@genentech need to be prepared to produce more doses of Actemra in collaboration with contract manufacturers and sell them at cost (~40 USD for 400mg vial) in LMICs... because a price tag of between 400-800 USD is way too high for many LMICs...
In order to further ramp up production of Actemra, @Roche/@Genentech should accept to put their cell line and manufacturing secrets in a technology pool. Then mAb producers around the world could access them and launch production of copies of Actemra.
The cell line access solution was proposed in this paper. Not my idea. But I support it in the case of tocilizumab, given the circumstances.
I’d like to know what regulators like @EMA_News & @US_FDA think about it.
f1000research.com/articles/7-537…
In summary:
For access to #tocilizumab for #COVID19:
- Lower price for LMICs, in line with cost of production.
- Accelerated approval of biosimilars.
- Put cell line & know how in a tech transfer pool.
Dear @Roche/@Genentech and regulators, please make it happen.