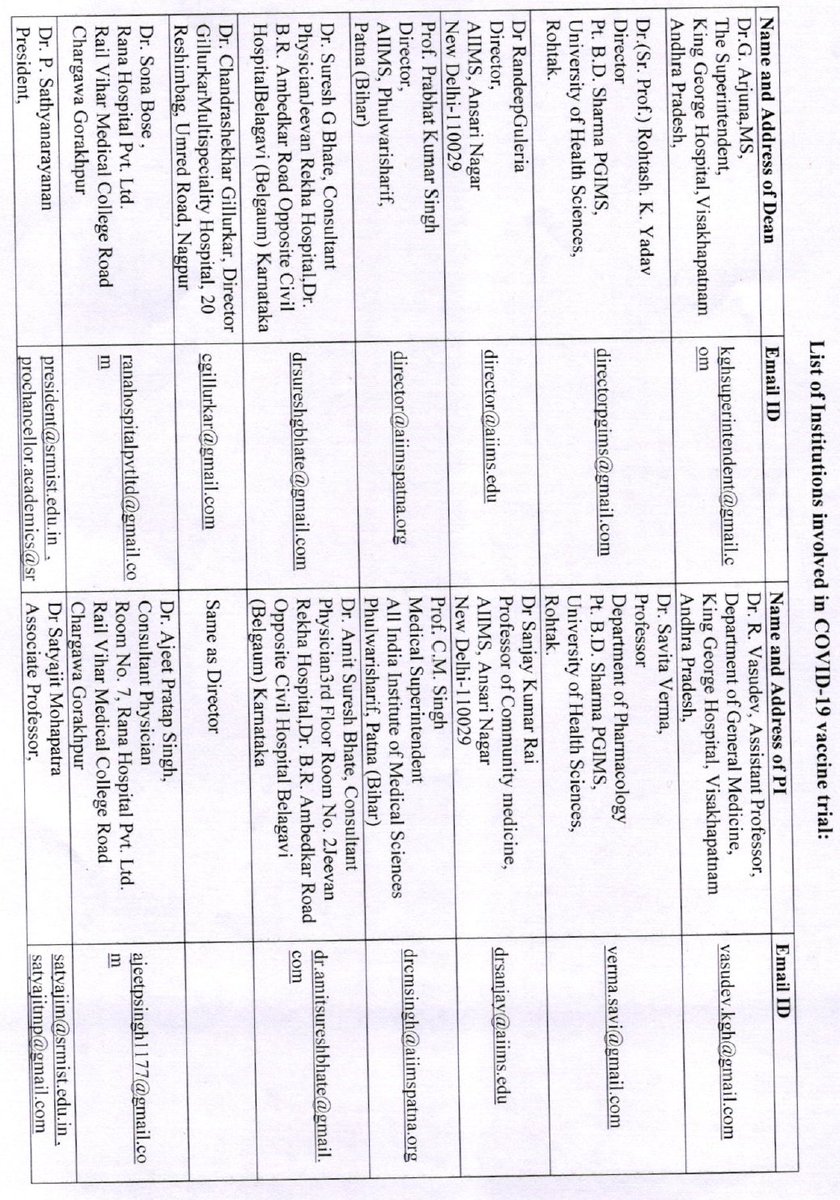
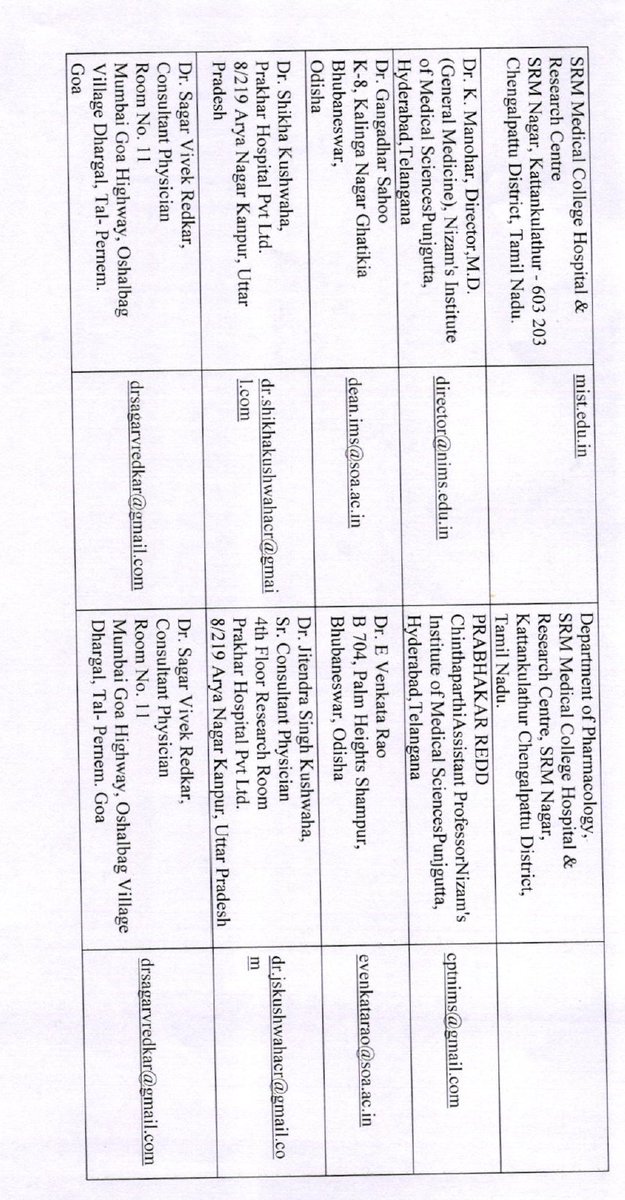
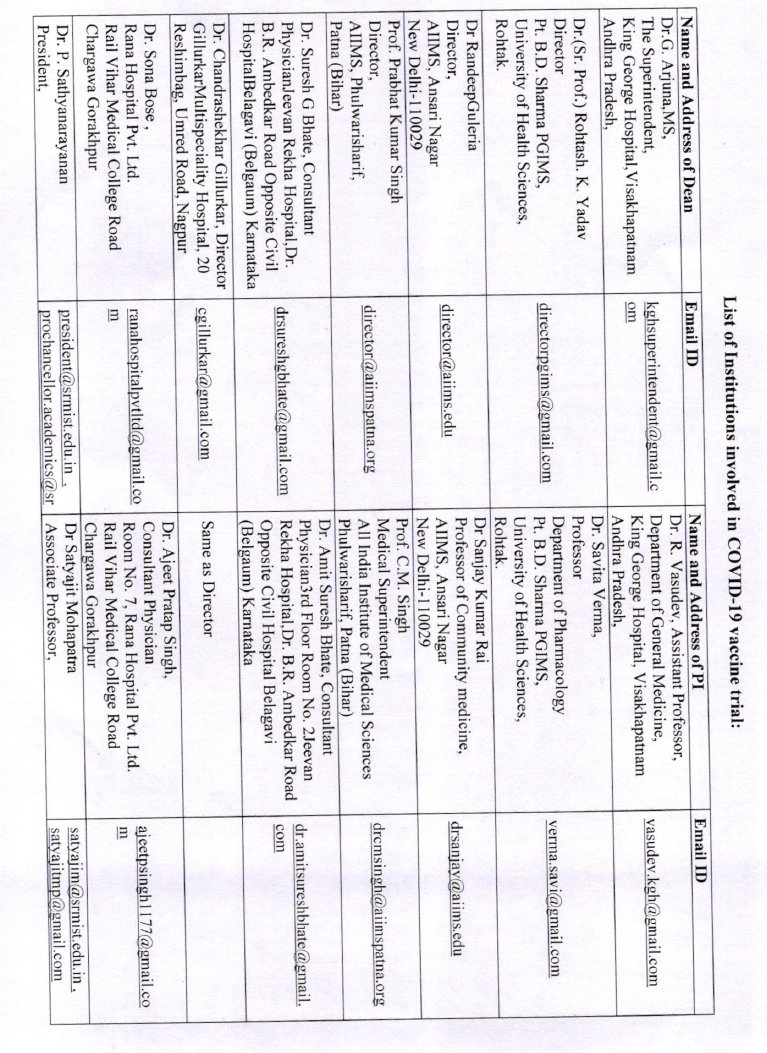
And that the vaccine will be launched on 15th August? A vaccine trial completed in little over a month, efficacy pre-decided?
Other wise non-compliance will be viewed seriously? By whom? by ICMR? under what power?
expresspharma.in/covid19-update…
ctri.nic.in/Clinicaltrials…
So of course ICMR as a co-creator of the vaccine is also having a conflict of interest in this study.
They are a co-creator of the vaccine.
Should they be writing to sites directly?