No doubt many of you heard of it, either on this platform, or on the news. It generated a lot of buzz in the media, and not all of it is true. Lets walk through these, and how to get it set up in your lab.
covidtrackerct.com/about-salivadi…
It is an RNA-extraction free, dualplex PCR for saliva to detect #SARS-CoV-2. It is meant to make sample collection and testing simpler and cheaper for large screening needs - like any "return-to" program.
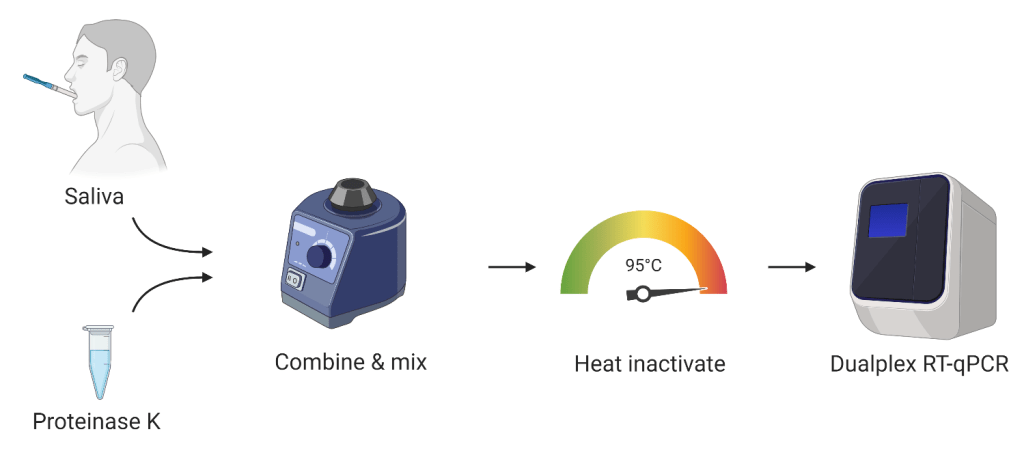
It isn't a point-of-care or an at-home rapid test. It uses PCR, and thus requires a high-complexity lab.
It's not a kit, it's a protocol – so we don't have products to distribute. Rather, for authorized labs, we can provide a protocol for doing inexpensive saliva-based RT-qPCR testing.
Only by authorized CLIA certified labs. At this moment we cannot help you to find or set up a lab to run SalivaDirect, as we are focused on authorizing labs that are currently CLIA certified.
If you represent a high complexity CLIA certified lab within the United States and would like to become authorized, please follow the steps listed on our website:
covidtrackerct.com/about-salivadi…
Automation. We are working with several institutions to validate robotic systems to automate the sample processing and/or PCR. Once these are validated, we'll add the protocols to our EUA for others to use.
Rapid detection. We are working on approaches to replace the PCR step with either LAMP or RPA. PCR will still have a role, but a simple rapid test has the ability to be point-of-care.
Pooling. By combining saliva from many people and testing it all at once, we can increase the number of samples that can be tested per day and reduce the amount of each reagent used per person.
covidtrackerct.com/jobs/