What if Lilly's monoclonal antibody trial was stopped due to futility?
1. A pause at 300 patients after 5 days of treatment was pre-specified in the protocol
nytimes.com/2020/10/13/hea… by @KatherineJWu @katie_thomas
1. A pause at 300 patients after 5 days of treatment was pre-specified in the protocol
nytimes.com/2020/10/13/hea… by @KatherineJWu @katie_thomas

2. Trials have stopping rules for 3 main reasons:
—futility (further enrollment would not show benefit)
—safety (the intervention appears to have a hazard that precludes further enrollment)
—efficacy (the efficacy evidence is overwhelming and it would not be ethical to proceed)
—futility (further enrollment would not show benefit)
—safety (the intervention appears to have a hazard that precludes further enrollment)
—efficacy (the efficacy evidence is overwhelming and it would not be ethical to proceed)
3. Since the pause was pre-specified and Lilly announced a "potential safety concern," we don't know yet if it was for futility or safety. So far there have not been safety issues disclosed for the monoclonal antibody programs w/ now thousands of patients enrolled (Ab or placebo)
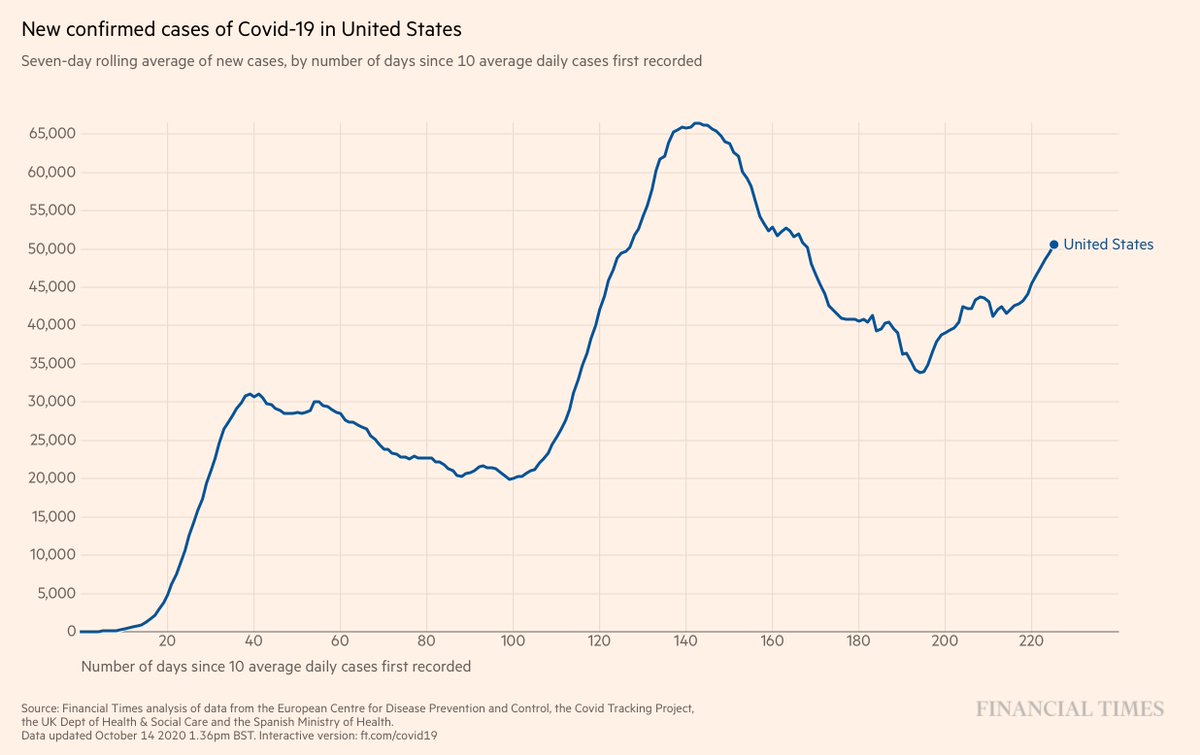
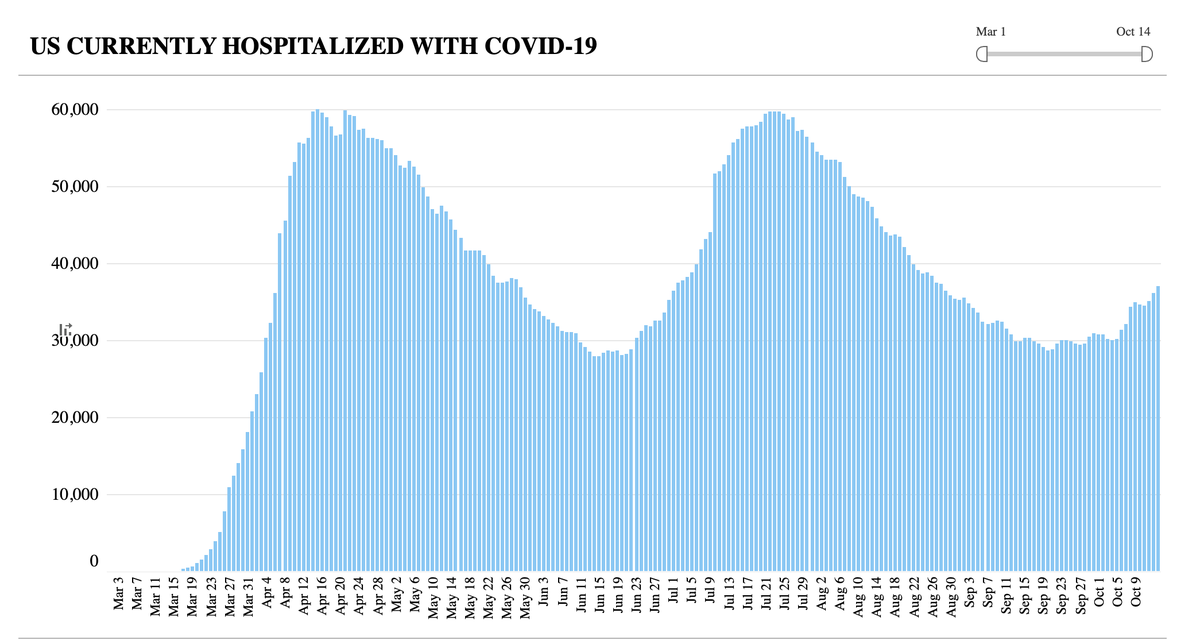
4. If the trial was stopped for futility, is that a sign that monoclonal antibodies aren't working?
To answer that, take into account the indication--hospitalized patients w/ advanced covid illness, all receiving remdesivir, too.
The Phase 2 trials in outpatients were encouraging
To answer that, take into account the indication--hospitalized patients w/ advanced covid illness, all receiving remdesivir, too.
The Phase 2 trials in outpatients were encouraging
5. Prior mAb studies in non-human primates indicate their capability to prevent illness given prophylactically, and to limit illness when given early after infection science.sciencemag.org/content/early/… @ScienceMagazine
6. So it could be that mAbs given too late are not helpful, perhaps even detrimental. This would be akin to what we've seen for dexamethasone and interferon to date. It could be a "timing is everything" story. We'll see. TBD 

• • •
Missing some Tweet in this thread? You can try to
force a refresh