There are two main ways to estimate the reproduction number for SARS-CoV-2, and I'd like to discuss the one that doesn't get so much attention... 1/
Most reported R values use a 'top-down' method, which estimates R from the growth pattern in various surveillance datasets (e.g.
https://twitter.com/AdamJKucharski/status/1259441472790724610?s=20), but there is also a 'bottom-up' method, which my @cmmid_lshtm colleagues have been using to track R... 2/
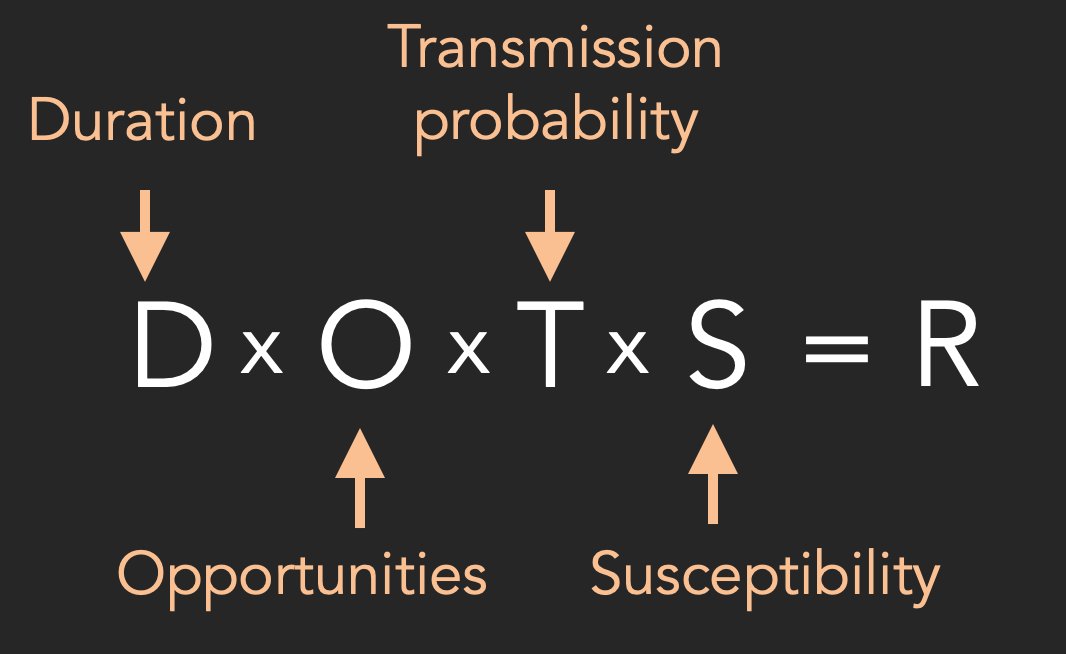
The basic idea is that R depends on four components: duration of infectiousness; opportunities for transmission (i.e. contacts); transmission probability during each opportunity; and population susceptibility... 3/ 

For respiratory infections that spread through face-to-face interactions, we can measure social contacts via surveys, and hence estimate how changes in 'opportunities' scale R. If average opportunities decline by a certain amount, so should the corresponding value of R. 4/
This is what my colleagues have tracked since March. After control measures were introduced, they estimated R declined to ~0.6 based on changes in conversational contacts. Soon after, this R<1 conclusion emerged in 'top-down' analysis of case data too. 5/
https://twitter.com/AdamJKucharski/status/1245248025146134530?s=20
Towards the end of the summer, contacts started increasing and so did corresponding estimates of R - again, this increase has also been reflected in observed surveillance data. 6/ 


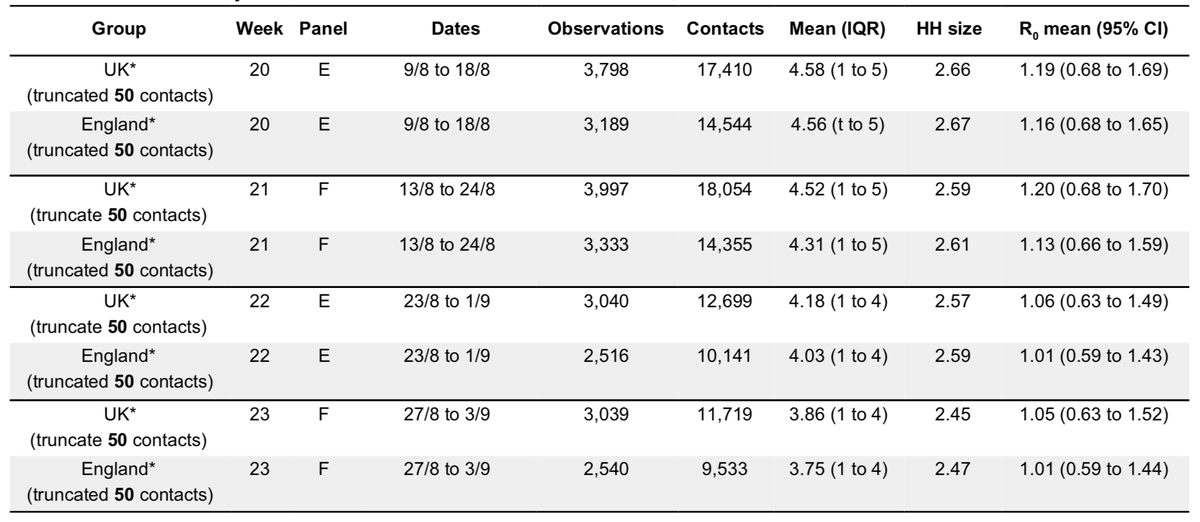
This approach is useful because it gives us clues about *why* R is changing, rather than just estimating by how much based on patterns in case data. It can also help us understand which contact patterns - and in which groups - are likely driving transmission 7/
Of course, ideally we'd eventually see a decoupling between social contacts and transmission, with reduction coming from testing & tracing (i.e. reducing effective infectious duration) or vaccine-induced immunity (reducing susceptibility), while allowing more interactions. 8/8 
• • •
Missing some Tweet in this thread? You can try to
force a refresh