NEW terrific study on rapid tests
The importance of this figure canNOT be overstated!
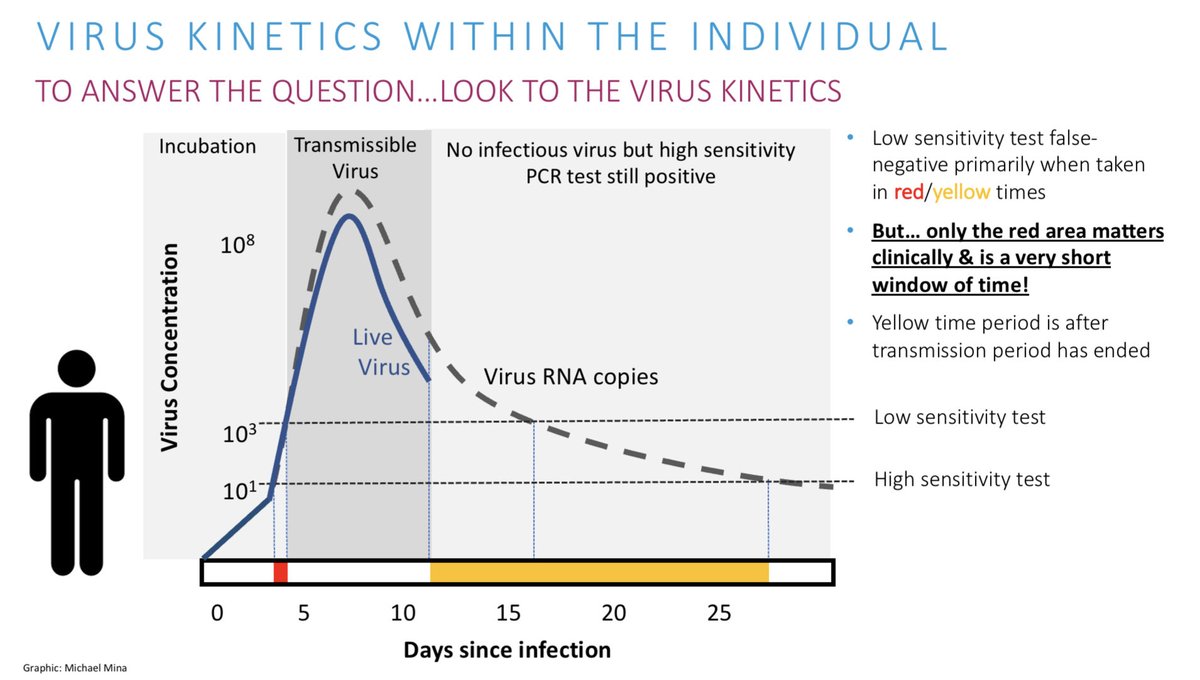
Rapid tests caught 80% of PCR positives but all missed had very low viral loads
Thus, as expected it will successfully capture ppl most likely to be contagious
1/
medrxiv.org/content/10.110…
The importance of this figure canNOT be overstated!
Rapid tests caught 80% of PCR positives but all missed had very low viral loads
Thus, as expected it will successfully capture ppl most likely to be contagious
1/
medrxiv.org/content/10.110…

The specificity of the PanBio, BinaxNow and SD Biosensor tests (the three leading manufactured rapid tests in the world) are looking very good!
In this paper, specificity was 100% in >400 samples... thus >99.2%
Now multiple studies showing very high specificity!
2/
In this paper, specificity was 100% in >400 samples... thus >99.2%
Now multiple studies showing very high specificity!
2/
The sensitivity metrics AND specificity metrics are now completely in alignment with what we have proposed for frequent rapid testing that can control outbreaks without vaccines.
medrxiv.org/content/10.110…
3/
medrxiv.org/content/10.110…
3/
Also note that in this study the Ct values are Very low on average. This drives home the message that Ct values must be calibrated per lab and per test. These are, overall, lower by 5 Ct values or so from other labs. Thus we can compare Cts but must take care to do it correctly.
• • •
Missing some Tweet in this thread? You can try to
force a refresh