A new article in @nytimes states rapid tests falter in asymptomatics
Warning of their use for asymptomatic screening
Says: among random asymptomatic #COVID19 screens, Rapid Tests caught only 32% of PCR +ve people
This is misleading...
1/
nytimes.com/2020/11/02/hea…
Warning of their use for asymptomatic screening
Says: among random asymptomatic #COVID19 screens, Rapid Tests caught only 32% of PCR +ve people
This is misleading...
1/
nytimes.com/2020/11/02/hea…
Tests have to be matched to their purpose. If doing asymptomatic screening - you are looking for INFECTIOUS people.
Importantly, MOST (~70%+) of the time someone is PCR +ve, they are POST-infectious!
2/
Importantly, MOST (~70%+) of the time someone is PCR +ve, they are POST-infectious!
2/
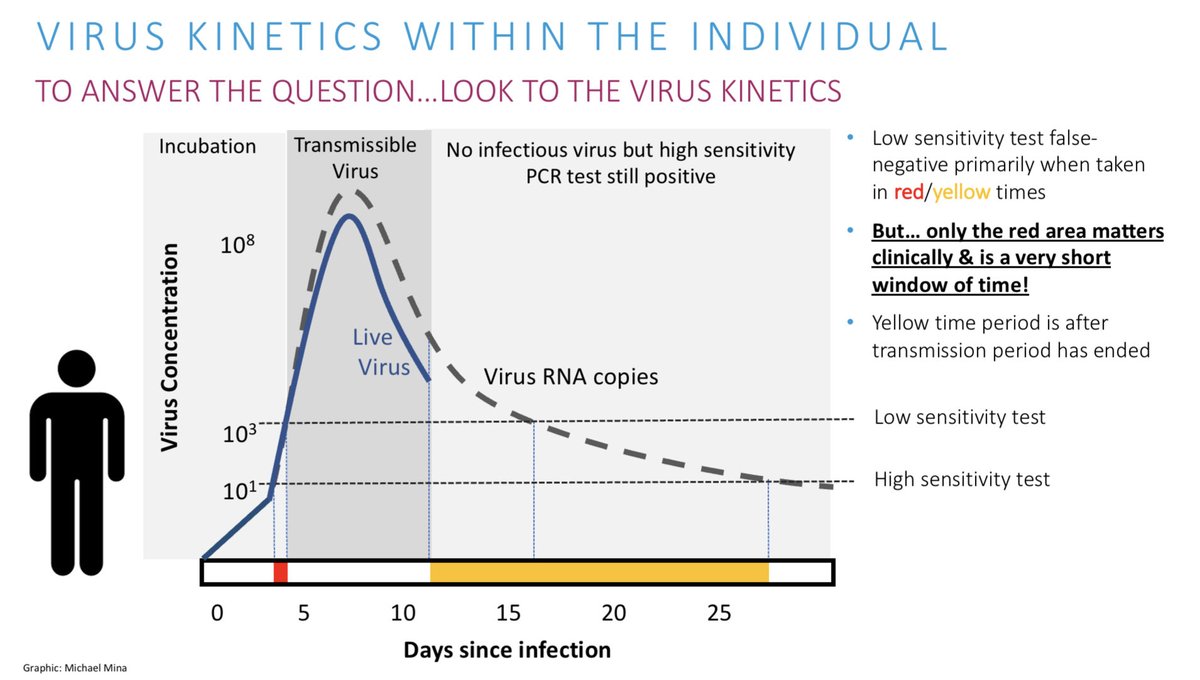
Since antigen tests meant to detect viable/live virus, we only EXPECT them to be positive about 30% of the time of PCR
Thus, finding 32% of positive PCR tests in random asymptomatic screening is absolutely the EXPECTED result for a test looking for infectious people
3/
Thus, finding 32% of positive PCR tests in random asymptomatic screening is absolutely the EXPECTED result for a test looking for infectious people
3/
In this same study the +ve PCR specimens that were NOT detected by antigen tests ALL had Ct values >31 and none had culturable virus!
Study after study after study now has demonstrated a lack of culturability as Ct values on most qPCR instruments get into the 30’s
4/
Study after study after study now has demonstrated a lack of culturability as Ct values on most qPCR instruments get into the 30’s
4/
We need to stop misinterpreting & causing concern about these tests.
The major tests appear to be performing exactly as expected.
This @nytimes piece should have stated in the headline:
“Asymtomatic screening for infectious ppl with antigen tests appears to be working”
5/
The major tests appear to be performing exactly as expected.
This @nytimes piece should have stated in the headline:
“Asymtomatic screening for infectious ppl with antigen tests appears to be working”
5/
But instead the headline was entirely misleading.
The article led w a comment from an ‘expert’ stating: “32% is a very low sensitivity. I’m surprised by how low that is.”
The expert is NOT a microbiologist, virologist, infectious disease physician, nor epidemiologist.
6/
The article led w a comment from an ‘expert’ stating: “32% is a very low sensitivity. I’m surprised by how low that is.”
The expert is NOT a microbiologist, virologist, infectious disease physician, nor epidemiologist.
6/
I know this is COVID times but if @nytimes is interviewing experts to write stories that have national and international implications for testing programs, why interview someone who is not an expert in the field. The 32% is not surprising - it is 100% expected - textbook.
7/
7/
I will say however that if you get past the headline and first few paragraphs, the rest of the article is spot on. It does eventually get to providing a balanced view of testing, which I appreciate....
8/
8/
Since the article 👆came out, I have had state, national and international leaders reach out asking if they need to stop their antigen testing programs if the tests only have 32% sensitivity.
This is not a time to grab attention w misleading headlines
9/
This is not a time to grab attention w misleading headlines
9/
To reiterate: the test worked 100% as expected for asymptomatic screening. It caught people with likely viable/transmissible virus and failed to catch people with unculturable virus / RNA detected.
I look forward to reading the paper when it is made available.
10/10
I look forward to reading the paper when it is made available.
10/10
Also, these nuances of Virus kinetics and PCR positivity vs antigen positivity vs transmissibility are NOT simple and are NOT the types of things, particularly w a novel virus, that most physicians and researchers are supposed to know. This is a niche area of science.
11/
11/
It is partly why there has been so much confusion. Most ppl do not study/model within-host virus kinetics and link to diagnostic and public health tools. It’s a small niche area
But for those of us who do study these pre-COVID, this is all turning out as expected - a good thing
But for those of us who do study these pre-COVID, this is all turning out as expected - a good thing
On original topic - Another way to put this is that these tests are simply faltering to detect virus in people who are recently recovered from infection. This is a good thing in many ways! If we want to detect people who have cleared the virus, we can use IgM antibody test
The reporter of the @nytimes piece wrote a nice thread on the article. Still starts off with what I think is the same wrong central message since we DO EXPECT the 32% results that were found. But like the article, if you dig in further, it is worth the read
@KatherineJWu
@KatherineJWu
https://twitter.com/katherinejwu/status/1323309527702319112
• • •
Missing some Tweet in this thread? You can try to
force a refresh