With Pfizer’s vaccine being granted authorization in the UK and the prospect of a Covid-19 vaccine EUA here in the US, I wanted to take a moment to answer some of the most common questions surrounding these vaccines. (1/16) 
“Why is it so tricky to distribute these vaccines?” It’s in large part because the two vaccines currently under EUA review have to be stored at super cold temperatures. (2/16)
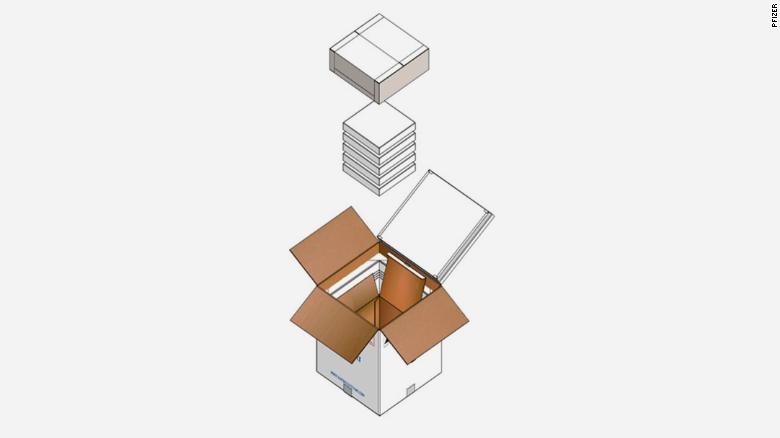
Pfizer's vaccine needs the coldest storage at -70C; -94F and that is unavailable in most places. Pfizer has even designed special “thermal shippers” to help transport its vaccine. (3/16) 
Moderna's vaccine requires storage at -20C; -4F. Also very cold, but doable in most hospitals. (4/16) 
Other vaccines like the one from Astrazeneca/Oxford or Johnson & Johnson can be stored at 2-8C; 36-46F. Much easier because a simple refrigerator will do. (5/16)
Another common question: "Who gets it first?” Vaccine advisers to the CDC voted yesterday to recommend that both health care workers (21 million) and residents of long-term care facilities (3 million) be vaccinated first. (6/16)
cnn.com/2020/12/01/hea…
cnn.com/2020/12/01/hea…
“What about the rest of us?” Access to the vaccine will continue to be rolled out in phases. Yesterday Lt. Gen. Paul Ostrowski of OWS said by June, “A hundred percent of Americans who want the vaccine will have had the vaccine by that point in time.” (7/16)
“Could getting vaccinated result in getting an infection?” The answer is no and that’s because there is no actual virus in the vaccine, only the genetic code for a portion of the virus. (8/16) 
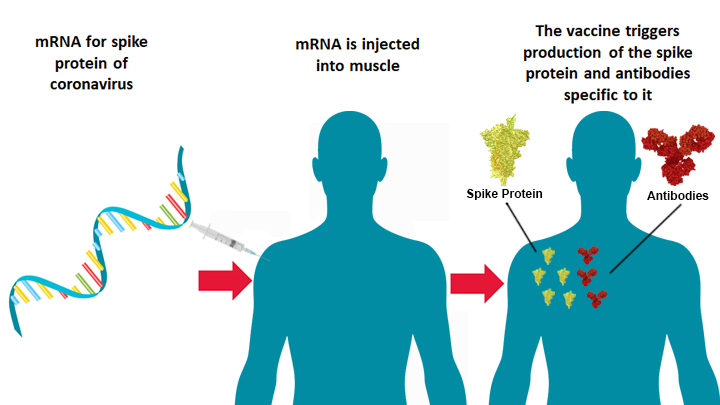
The reason some people feel crummy after is because the vaccine is revving up the immune response. (9/16)
cnn.com/2020/12/02/hea…
cnn.com/2020/12/02/hea…
“Is it safe?” Both the Pfizer and Moderna vaccine needed to meet a two-month safety threshold before applying for an EUA, but long-term safety of the vaccines is still unknown. (10/16)
"I always make sure I say that while we know that the short-term – and I'm going to call it mid-term – safety of this vaccine is now well understood, the very long-term safety is not yet well understood by definition," Moncef Slaoui said Tuesday. (11/16)
washingtonpost.com/washington-pos…
washingtonpost.com/washington-pos…
“Will I need to get the vaccine if I’ve already had Covid-19?” The answer: probably. (12/16)
The idea behind a vaccine is it should stage a stronger (and maybe longer) immune response than your body has after a natural infection. We still don’t know how long “natural immunity” from a Covid-19 infection might last. (13/16)
And finally, “Did Moderna really create a vaccine in just two days?” Yes and no. The mRNA platform Moderna uses for its vaccine was developed over many years. But the design for the Covid-19 vaccine was done just in two days. (14/16) 

However, if this platform hadn’t already been developed, that two-day timeline would have been impossible. (15/16)
cnn.com/videos/health/…
cnn.com/videos/health/…
As we learn more about the vaccines, we’ll continue to answer your questions. In the meantime, stay safe and #BeKind . (16/16)
cnn.com/video/data/2.0…
cnn.com/video/data/2.0…
• • •
Missing some Tweet in this thread? You can try to
force a refresh