It's Thursday and time for toxicology, so enough politics, let's talk poisons (I think I need this more than you!). Today it's all about one of our favorite condiments, HORERADISH. It may seem simple, but its pungency is due to a binary weapon! 

So that's horseradish growing in my garden, in case you were wondering where it actually came from. It goes amazingly well and the young leaves are edible - they add a nice little kick to a sandwich. 

But horseradish sauce doesn't come from leaves, it's made from horseradish roots, like these that I harvested: 

If you've had horseradish sauce, you know it comes in various forms of pungency. Some can be mild, some induce lachrymation (fancy word for "causing tears"), some "burn" your mouth. This is due to a chemical called allyl isothiocyanate - nasty stuff. 
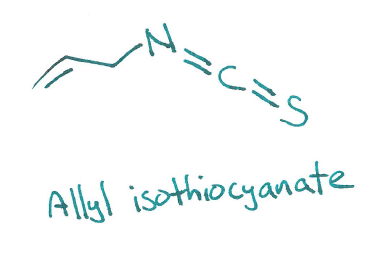
Ally isothiocyanate works on TRPA1 receptors. These are cousins of the TRPV1 receptors activated by capsaicin in chili peppers, so you can see how the "spiciness" of horseradish is related.
The funny thing though? Horseradish doesn't really contain ally isothiocyanate. Yeah, I know what you're thinking.
Don't bail on me yet, I'll explain. Horseradish contains sinigrin, a glucosinolate found in a lot of vegetables in the Brassicaceae family, like Brussels sprouts and broccoli. 

Horseradish also contains an enzyme, myrosinase. So when an animal comes up to horseradish and has a nibble, the cells break open and release sinigrin and the enzyme myrosinase, what happens next is pure (bio)CHEMISTRY!
Myrosinase catalyzes the conversion of sinigrin to allyl isothiocyanate at neutral-ish pH. So when you chew on a leaf you're generating ally isothiocyanate IN YOUR MOUTH. You're literally a walking science experiment. 

So when you make horseradish, you grind up those roots, releasing siningrin and myrosinase, and generating ally isothiocyanate. Pretty cool, but wait, there's more!
How do you get varying levels of "hotness?" Time and pH. The longer you let horseradish sit - a few minutes is all it takes - the more allyl isothiocyanate is made, and the more pungent the sauce. To stop the formation, vinegar is added, which converts sinigrin to allyl cyanide
This is something you can do yourself, too! Growing horseradish is ridiculously easy from planting a root - but be careful, it can grow out of control, which is why mine is in a large pot. Then harvest the roots after a year or two. 

Grind it all up and wait a few minutes, then add vinegar once the desired hotness" is reached to stop the chemical reaction. I will warn you though, if you do large batches, consider doing it outside - I nearly killed my dad one time. 

So that's the chemistry and science of horseradish. Now go forth and annoy all your friends and family about sinigrin and allyl isothiocyanate the next time you have roast beef or a Passover meal. Be good, people, and science on. 

If you want the story of how I nearly killed my Dad with horseradish, with the famous line of "because I’m not only a scientist, but also kind of a dick," you can read about it here: naturespoisons.com/2014/12/10/the…
• • •
Missing some Tweet in this thread? You can try to
force a refresh