THE VACCINE DILEMMA
The government has announced an intent to go ahead with vaccination as of 16th Jan 2021 with both Covishield and Covaxin. There is going to be no choice. You will only be able to receive one of the two, whatever is allotted to you by the govt.
The government has announced an intent to go ahead with vaccination as of 16th Jan 2021 with both Covishield and Covaxin. There is going to be no choice. You will only be able to receive one of the two, whatever is allotted to you by the govt.
While you have an option of opt-out, it will put potential vaccinees in a tough situation. You have to decide for yourselves based on publicly available (or the lack fo it) information-- on safety, immunogenicity, and efficacy on both vaccines.
If you are allotted Covishield, you will follow the current protocol as described and practiced in the dry runs. 

Additionally, you will receive this fact-sheet as a recipient
for #COVISHIELD prepared by the company.

for #COVISHIELD prepared by the company.


Remember there still is concern about (lower) advocated efficacy of Covishield with the current advocated spacing between the doses in India. @LeroyLeo7 has covered this for @livemint today
livemint.com/companies/news…
livemint.com/companies/news…
Now what if you are supposed to get Covaxin. We know the phase 3 trials are not yet over, only data available is on safety and immunogenicity. And the approval for Restricted Emergency Use is in clinical trial mode. What that means is still not completely clear.
But some details now emerging. Here are the minutes of the 137th meeting held on 12 Jan 2021 for COVID-19 related proposals- there is a protocol which apparently has been cleared by ICMR CECHR, an Informed consent form, adverse event form, factsheet. #Covaxin 
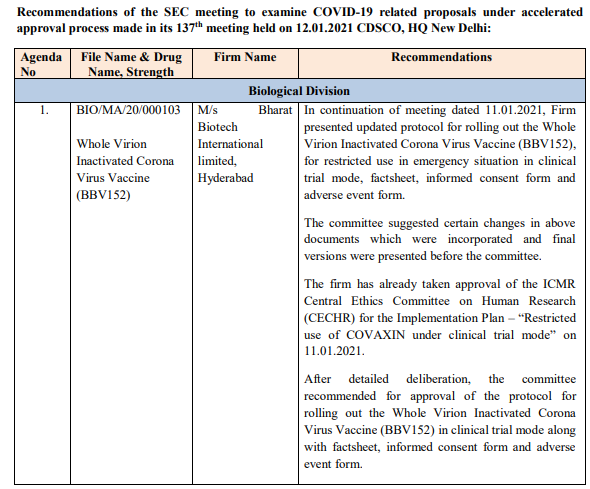
Interestingly as of now the minutes of the previous meeting (136th meeting) held on 11 Jan 2021 are not yet available on the website. 

Here is apparently the product insert for Covaxin
https://twitter.com/AnantBhan/status/1349755700809928706
So, if you are in the Covaxin arm, you are supposed to be ADDITIONALLY also administered informed consent which by nature is voluntary and requires you to be given time if you want to decide. Good consent also requires time. Has this been planned in vaccination roll out?
Let's say for good quality consent, you need to spend an average of AT LEAST 30-45 minutes. For 100 vaccine recipients which is the intended number at each vaccination centre per day, that should take 3000 minutes in total. Will there be separate dedicated staff for that?
Has this additional time/staff for consent been factored in? Remember you need to also have privacy provided for the consent process-- is there a dedicated room planned for thi at the vaccination sites?
#clinicaltrial #mode
#clinicaltrial #mode
Will there be a Principal Investigator? Will there be an ethics committee providing oversight? What does the protocol say?
Also, will you be covered for medical care and compensation if there is an adverse event with causality established as is usually done in a clinical trial?
Also, will you be covered for medical care and compensation if there is an adverse event with causality established as is usually done in a clinical trial?
The usual requirement is to register a clinical trial prior to recruiting your first participant on the Clinical Trials Registry of India
No entry yet on this (?) new clinical trial in CTRI. Is this being skipped?
No entry yet on this (?) new clinical trial in CTRI. Is this being skipped?
Additionally, remember informed consent is a process, not just the documentation on the Informed Consent Form. So those who get the vaccine (first dose) will need to be updated ethically in due course about any information on the vaccine (efficacy data?) which becomes available.
Now, this might be prior to the second dose. So, then they will have a choice to decide about continuing in this 'clinical trial'.
Now given this, you might wonder, would this requirement also not apply to those participants who are part of the ongoing Covishield phase 2/3 safety-immunogenicity study and the Covaxin phase 3 Efficacy, Safety, Immunogenicity,and Lot-to-Lot consistency study #bioethics
Bingo, you are right. Ethics would require that these participants be informed immediately that two vaccines have been given Restricted Emergency Use Approval in India- they could be in a clinical trial for one of these. And that they have a choice of withdrawing from the study
This is going to be relevant because many of these participants are health workers, frontline workers,& could be eligible for the government vaccination program in the first couple of waves.
It would be ideal if they continued in the trials they are part of, but its their choice
It would be ideal if they continued in the trials they are part of, but its their choice
Would some of them not knowing whether they got vaccine candidate or placebo in the Covishield or Covaxin clinical trial want to not take chances-- and take the vaccine being offered through govt roll-out-- yes certainly a possibility.
Question is-- have they been told yet about the approvals-- though many of them would presumably have heard through the news/media, you still need to formally inform as part of ethical obligations.
Is there a requirement to stop the studies and unblind- probably not yet.
Is there a requirement to stop the studies and unblind- probably not yet.
We have discussed this issue in a @WHO Policy brief
Emergency Use Designation of COVID-19 candidate vaccines: Ethical considerations for current and future COVID-19 placebo-controlled vaccine trials and trial unblinding
apps.who.int/iris/bitstream…
Emergency Use Designation of COVID-19 candidate vaccines: Ethical considerations for current and future COVID-19 placebo-controlled vaccine trials and trial unblinding
apps.who.int/iris/bitstream…

Moving forward for future vaccine candidates, does the availability of these vaccines through government roll out constitute a new standard of care? And hence makes placebo-controlled clinical trials unethical?
Remember comparator clinical trials (new vaccine candidate Vs a vaccine candidate rolled out) will require higher sample size, be more expensive to do, and require more time.
This will of course depend on what we consider Standard of Care.
This will of course depend on what we consider Standard of Care.
Should vaccines which have received Restricted Emergency Use/EUA approval be considered to constitute the new Standard of Care?
We do speak to this issue in the policy brief
apps.who.int/iris/bitstream…
We do speak to this issue in the policy brief
apps.who.int/iris/bitstream…

This issue also raised in detail by @RPrasad12 here
https://twitter.com/RPrasad12/status/1349801299139825664
Here is the consent form for Covaxin via @SumitraRoyTOI
https://twitter.com/SumitraRoyTOI/status/1350277480226033664?s=19
Important for everyone to remember that the government through the ICMR is a co-sponsor for the clinical trials for both vaccines, Covishield and Covaxin in India and hence has stake also in the vaccine research process and the data produced thereof.
Remember above that the vaccine is NOT mandatory. And that there is opt out and the government has also said so. Of course though local leadership might make their own worrying interpretations
See example below. We might see more of this if uptake is low. Hope no target setting
See example below. We might see more of this if uptake is low. Hope no target setting
Letter by the Civil Surgeon (equivalent to CMO/CMHO) in Koderma in Jharkhand mandating local govt health workers to take Vaccine for COVID-19, or otherwise their salary will be withheld. 

Such events will happen in the duration of large vaccination campaigns. Important to identify quickly and provide medical attention as done in this case.
But since Covaxin is rolled out in 'clinical trial mode', should this be called SAE or an AEFI?
But since Covaxin is rolled out in 'clinical trial mode', should this be called SAE or an AEFI?
https://twitter.com/Milan_reports/status/1350479201426571264?s=19
Some of you if you have the time might want to listen to the presentations and discussions in this webinar
https://twitter.com/AnantBhan/status/1350819875397140481
Very relevant points by Prof Shahid Jameel in his interview here
https://twitter.com/RemaNagarajan/status/1350683011335917568
Need for nuance in our understanding of the current situation
timesofindia.indiatimes.com/home/sunday-ti…
timesofindia.indiatimes.com/home/sunday-ti…

There have been some teething issues with the backbone of the vaccination efforts-- server issues etc for #CoWIN as reported in the media. The app by itself seems to be work in progress as evidenced by the call by MeITY to improve the platform (now extended to 18th Jan 2021) 



The seven problem statements identified for COWIN improvement are very interesting. Glad to see that innovation and Quality Improvement is being considered crucial as the vaccination roll out continues
app.thebizplanner.com/public/applica…

app.thebizplanner.com/public/applica…


This is not very reassuring!
https://twitter.com/OfficialSauravD/status/1351070826666352644
Now this recruitment ad for Zydus-Cadila's COVID19 #vaccine Zycov-D as received as forward on WA
How can we promise "high efficacy and safety" when phase 3 trials are ongoing?
How can we promise "high efficacy and safety" when phase 3 trials are ongoing?

When trade secret, commercial interests trump public interest and the confidence in the regulatory process
https://twitter.com/OfficialSauravD/status/1352253333730258944?s=19
Covaxin is being rolled out in "clinical trial mode", a key part of which is informed consent being administered prior to vaccination
Except some health workers are saying consent not sought in some sites
Except some health workers are saying consent not sought in some sites
https://twitter.com/ranjanikanth_/status/1352463113602260994?s=19
• • •
Missing some Tweet in this thread? You can try to
force a refresh








