RECOVERY Update:
30,000 patients now enrolled in one country in 10 months.
The most extraordinary effort.
And still ongoing.
30,000 patients now enrolled in one country in 10 months.
The most extraordinary effort.
And still ongoing.

In the words of the independent Data Monitoring Committee:
"Thank you for your continuing help and support."
"RECOVERY is a beacon of light in these difficult times."
"Thank you for your continuing help and support."
"RECOVERY is a beacon of light in these difficult times."

Four results now published and changing practice:
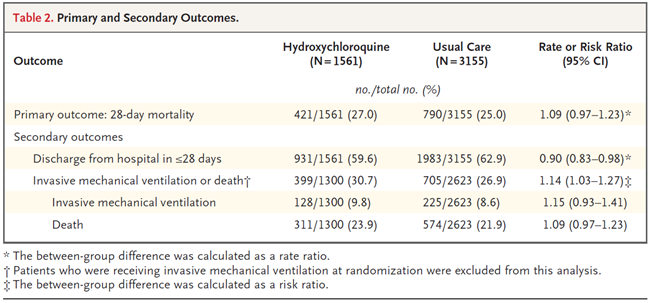
Hydroxychloroquine, Lopinavir-ritonavir, and Azithromycin ineffective - so no longer used.


Hydroxychloroquine, Lopinavir-ritonavir, and Azithromycin ineffective - so no longer used.



...and dexamethasone shown to reduce mortality for patients requiring oxygen or artificial ventilation.
A complete game-changer, and saving hundreds of thousands of lives.
A complete game-changer, and saving hundreds of thousands of lives.

With further results to follow:
Convalescent plasma (11,500 patients; now stopped; no effect on mortality)
Tocilizumab (3,800 patients ongoing)
REGN-COV2 monoclonal antibody combination (4,700 patients ongoing)
Aspirin (8,600 patients ongoing)
Colchicine (5,800 patients ongoing)
Convalescent plasma (11,500 patients; now stopped; no effect on mortality)
Tocilizumab (3,800 patients ongoing)
REGN-COV2 monoclonal antibody combination (4,700 patients ongoing)
Aspirin (8,600 patients ongoing)
Colchicine (5,800 patients ongoing)
Why do we need randomised trials?
To discover which of the many promising treatments actually save lives and improve outcomes for patients
- some treatments will work - and can then be widely used
- some treatments will not - and can then be abandoned
To discover which of the many promising treatments actually save lives and improve outcomes for patients
- some treatments will work - and can then be widely used
- some treatments will not - and can then be abandoned
And why do trials need to be so big?
- to distinguish treatments with modest benefits (e.g. reducing risk of death by one-fifth) from those with no benefit
- and for those that work, to understand how well they work and in whom do they work (critical for implementation)
- to distinguish treatments with modest benefits (e.g. reducing risk of death by one-fifth) from those with no benefit
- and for those that work, to understand how well they work and in whom do they work (critical for implementation)
Many thanks to all our funders and supporters, including @The_MRC @NIHRresearch @HDR_UK @NHSDigital @wellcometrust @OxfordBRC and many more across the whole UK.
COVID has played a devastating toll on patients, their families, colleagues, communities, & wider society.
Behind the numbers are so many personal stories.
We are humbled by the contribution of all involved.
Together we have made a difference - and will do so again.
Thank you
Behind the numbers are so many personal stories.
We are humbled by the contribution of all involved.
Together we have made a difference - and will do so again.
Thank you
• • •
Missing some Tweet in this thread? You can try to
force a refresh