Wonderful news about the Johnson&Johnson vaccine! Let me explain (and also let me tell you I was just on an interview with Dr. Paul Offit, who believes - like me- that control is nigh). J&J vaccine press release here:
jnj.com/johnson-johnso…
jnj.com/johnson-johnso…
J&J vaccine is a modified cold virus adenovirus (this vector doesn't replicate or cause illness in humans) with double stranded DNA coding the spike protein of SARS-CoV-2 inside. Nice explanation of NYT of how it works. The adenovirus "vector" (carrier)
nytimes.com/interactive/20…
nytimes.com/interactive/20…
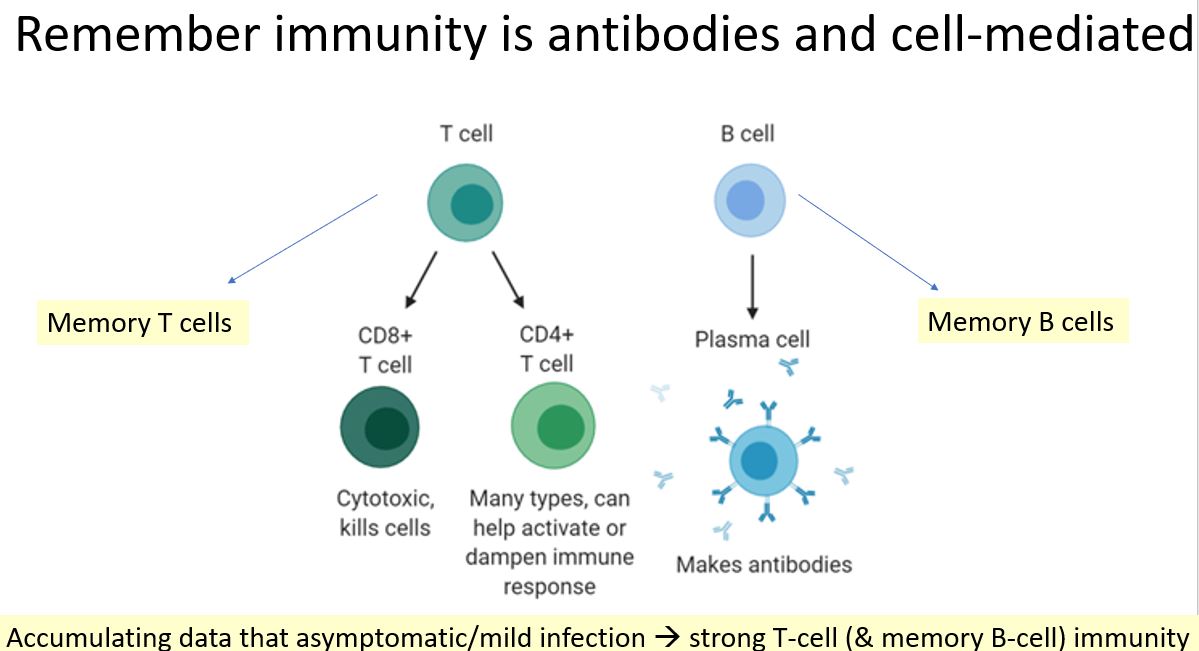
gets the DNA inside host cell nucleus where it is "transcribed" into mRNA and then you make the spike protein of the virus % raise immune response against it. Initial phase I/II data from NEJM showed high immunogenicity of 1st dose that went up over time.
nejm.org/doi/10.1056/NE…
nejm.org/doi/10.1056/NE…
Phase 3 trial (ENSEMBLE) enrolled 43,783 participants U.S./Latin America/S. Africa- outcomes: total 468 symptomatic cases of COVID-19 (some in placebo; some in vaccine) and let's breakdown 3 endpoints. 1st outcome of hospitalization/death- vaccine was 100% effective in preventing
100% effective against scariest thing- hospitalization/death. 2nd outcome. 2nd outcome of severe disease - vaccine was 85% effective against all regions. That is exactly what we don't want - severe disease. Also, immunogenicity phase I/II data showed increase in antibodies over
time with just 1 dose and trial looked at 14 days; 28 days after 1 dose so efficacy data likely to go up over time as your immune response escalates. Moderate disease outcome varied by region: Protection 72% in U.S., 66% in Latin America & 57% in South Africa at 28 days
Bottom line? 100% effective against the worst thing (hospitalization/death); 85% effective against severe disease; varying efficacy across regions against moderate disease. highest (72%) U.S. 1 dose; can be in fridge (regular fridge) for LONG time; 2-dose trial going. GREAT NEWS!
Want to clarify outcomes in the J&J vaccine trial: severe COVID-19 (lab confirmed) and >=1 of signs consistent with severe systemic illness, admission to ICU, shock, organ failure or death, among others. So kind of vague but hospitalization/death is a SUBSET of that so 100%
prevented that subset. Moderate- evidence of pneumonia, deep vein thrombosis (clot), shortness of breath (oxygen saturation>93%), respiratory rate ≥20; or >=2 systemic symptoms suggestive of COVID-19. So, as you can see severe would include O2 sat drop<93% (and could be once)
Also, please remember outcomes in phase 3 trial measured 14-28 days after 1st dose. Immunogenicity data (phase I/II trial) in NEJM shows increasing antibodies/immunity after 14-28d so 1 dose likely will work better after time (and 2-dose trial continuing):
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…
• • •
Missing some Tweet in this thread? You can try to
force a refresh