1. The B.1.1.7 variant could prove to be our last major obstacle to achieving containment of the virus in the US. But it isn't getting enough respect. Many states are eliminating their effective mitigation measures such as mask mandates and on gatherings.
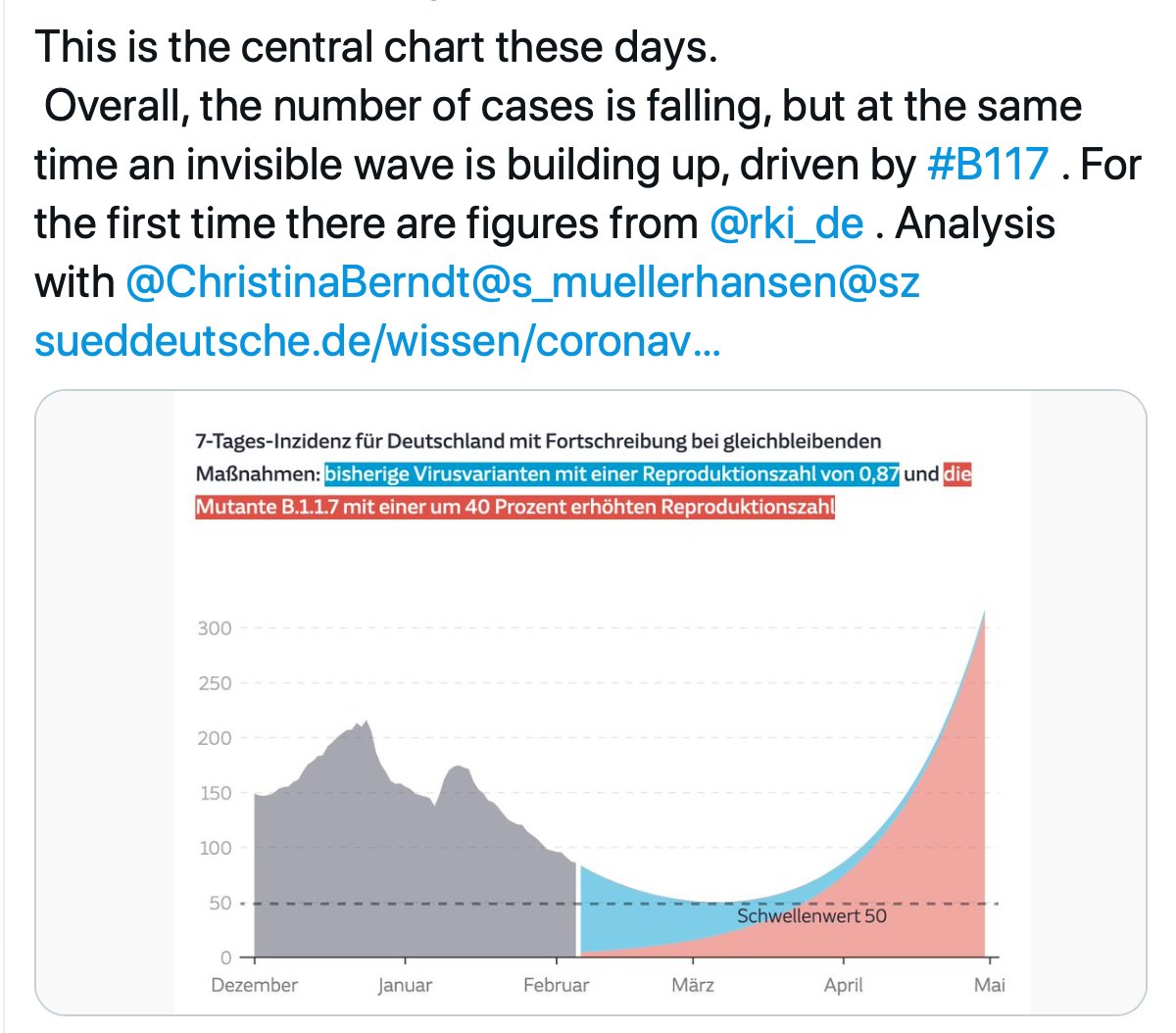
2. This variant increases 70-100% every week to crowd out other lineages and become dominant. In Germany, it is presently 5.8% (like San Diego) and here is their projection (via @kakape).
We are in the lull zone.
We are in the lull zone.
3. @CarolineYLChen talked with 10 experts
propublica.org/article/why-op…
@svscarpino @alliblk @datcummings @cmyeaton @Dr_KFO @PhilFebboMD_CMO @mtosterholm @larrybrilliant @llborio
TLDR "It’s really hard to thread this needle without sounding like a prophet of doom" —@angie_rasmussen
propublica.org/article/why-op…
@svscarpino @alliblk @datcummings @cmyeaton @Dr_KFO @PhilFebboMD_CMO @mtosterholm @larrybrilliant @llborio
TLDR "It’s really hard to thread this needle without sounding like a prophet of doom" —@angie_rasmussen
4. Is B.1.1.7 a variant or a strain?
latimes.com/science/story/…
It's behavior is markedly different from D614G. It clearly qualifies as a strain. While we're at it, on this #SuperBowlWeeknd, it is surely a superspreader strain since it is unequivocally more transmissible (≥50%)
latimes.com/science/story/…
It's behavior is markedly different from D614G. It clearly qualifies as a strain. While we're at it, on this #SuperBowlWeeknd, it is surely a superspreader strain since it is unequivocally more transmissible (≥50%)
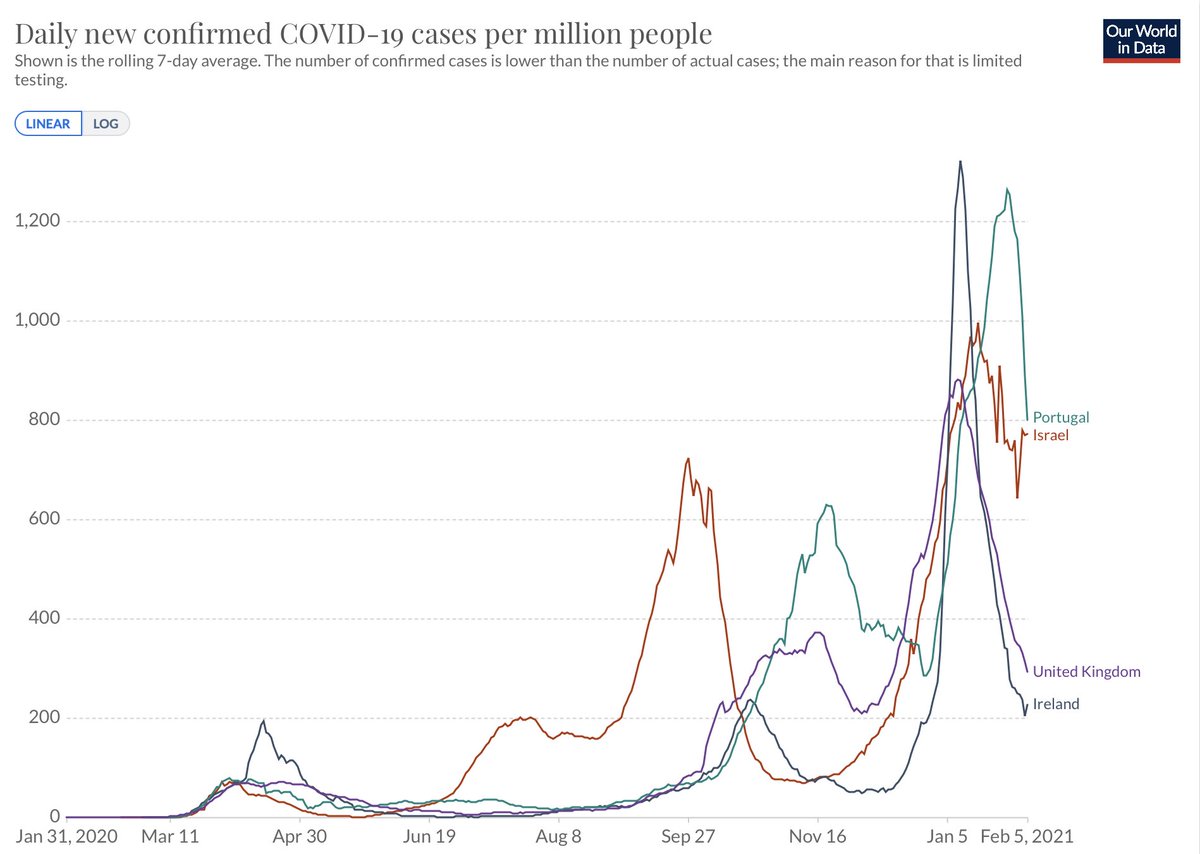
5. Instead of the Sturgis rally or Rose Garden superspreader events, now the virus is driving its dissemination beyond what people do. That's a worst case scenario. We have seen the toll it has taken in the UK, Ireland, Israel, and Portugal @OurWorldInData 
6. B.1.1.7 is here, in every state by now, and it is destined to do the same, in a patchwork pattern. We can't/ haven't vaccinated fast enough to put it into check. Relaxing mitigation now is the wrong strategy. It's denialism (like a year ago), the opposite of what we need to do
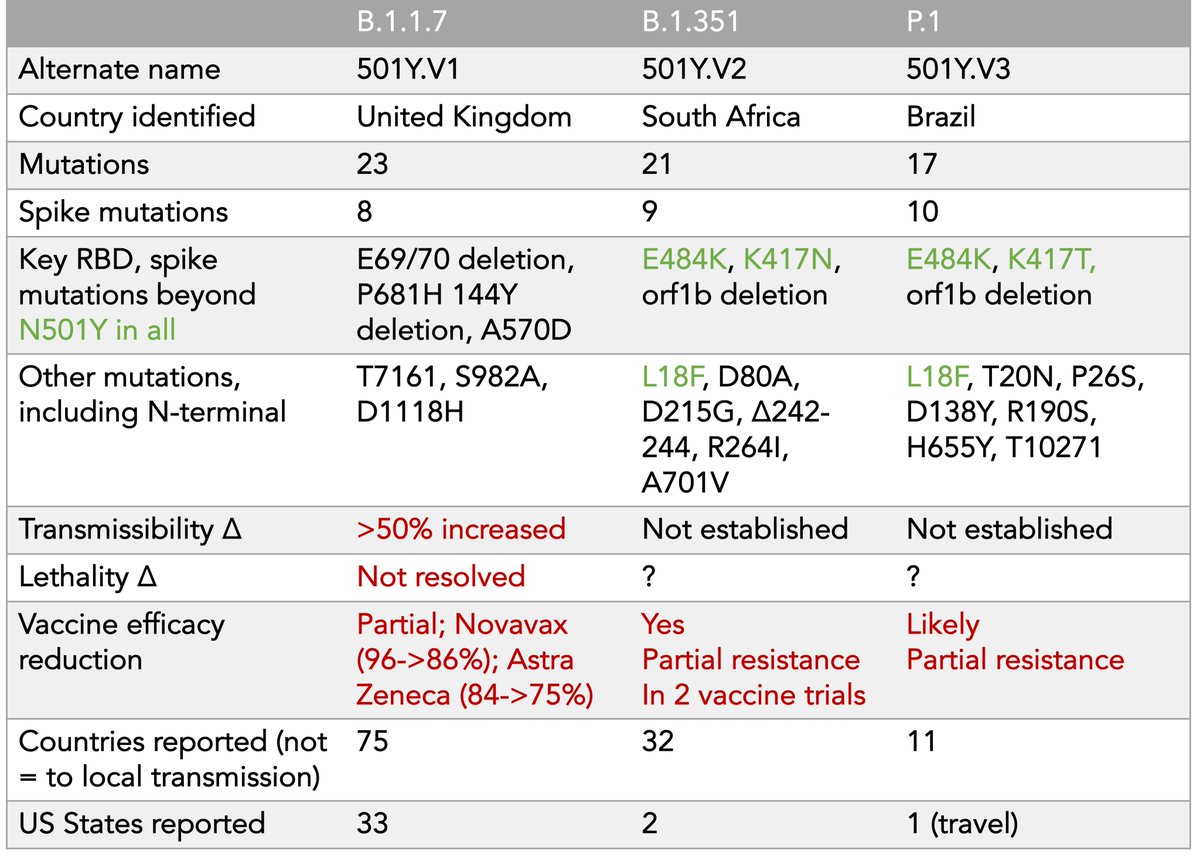
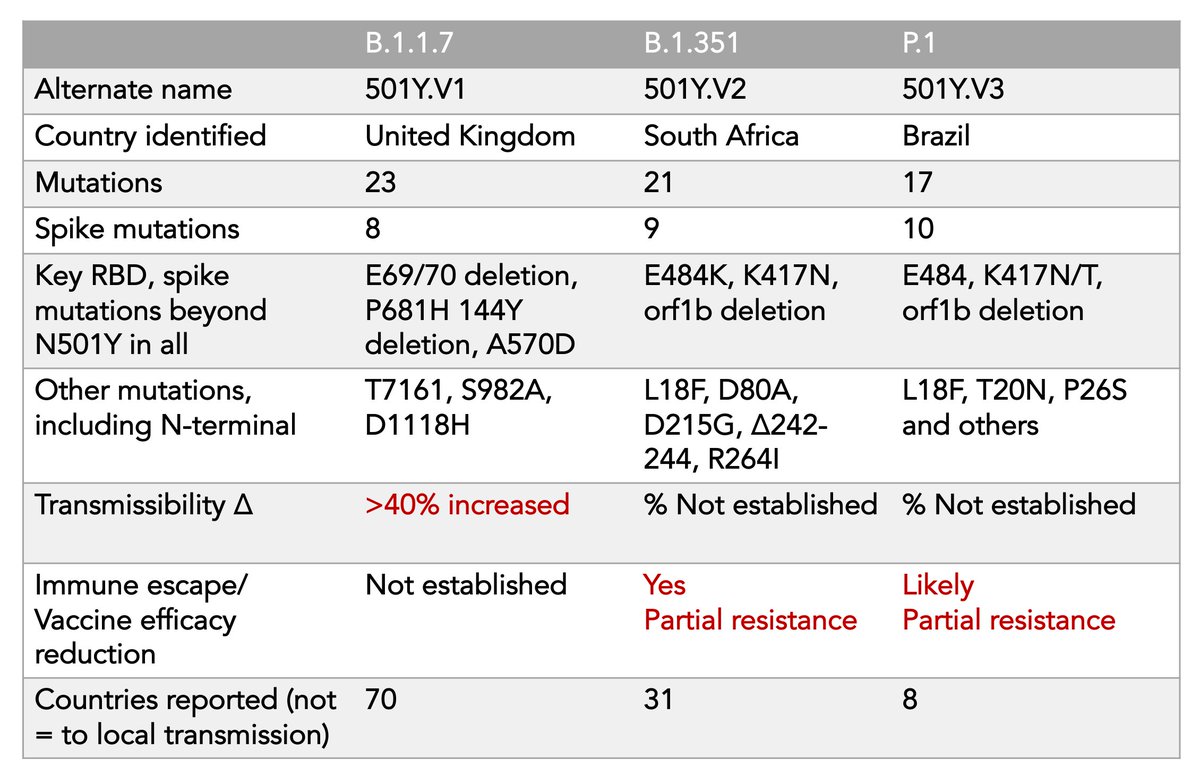
7. A bit more on B.1.1.7.
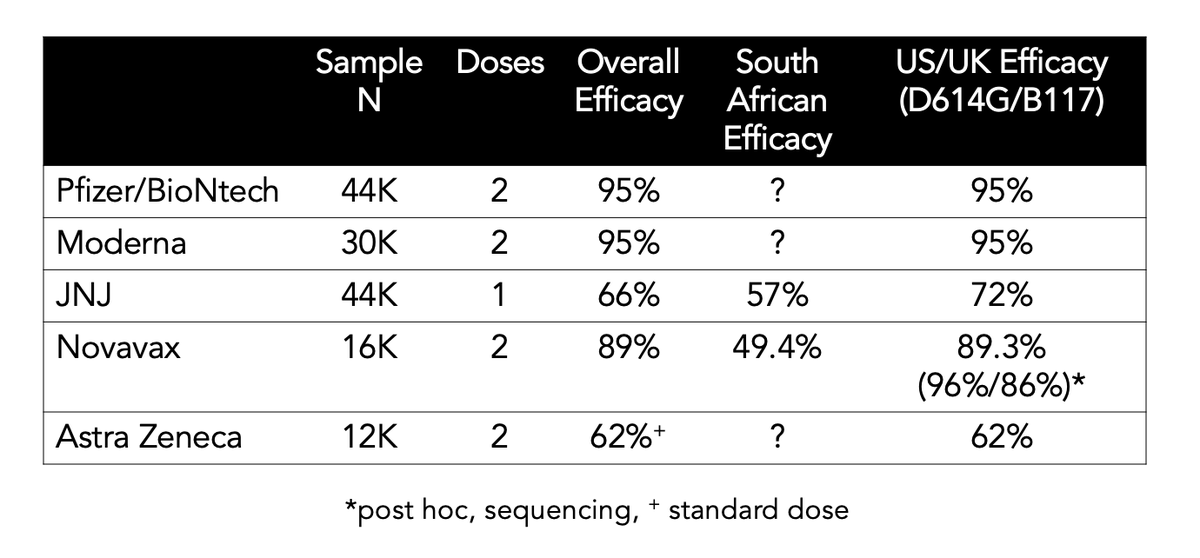
Its immune escape appears to be much less than B.1.351 or P.1; the vaccines are working, but:
Now 2 vaccine trials have shown a drop-off in efficacy compared with the ancestral strain
Novavax 96->86%; Astra Zeneca 84->75%

Its immune escape appears to be much less than B.1.351 or P.1; the vaccines are working, but:
Now 2 vaccine trials have shown a drop-off in efficacy compared with the ancestral strain
Novavax 96->86%; Astra Zeneca 84->75%
https://twitter.com/EricTopol/status/1357372875125104645

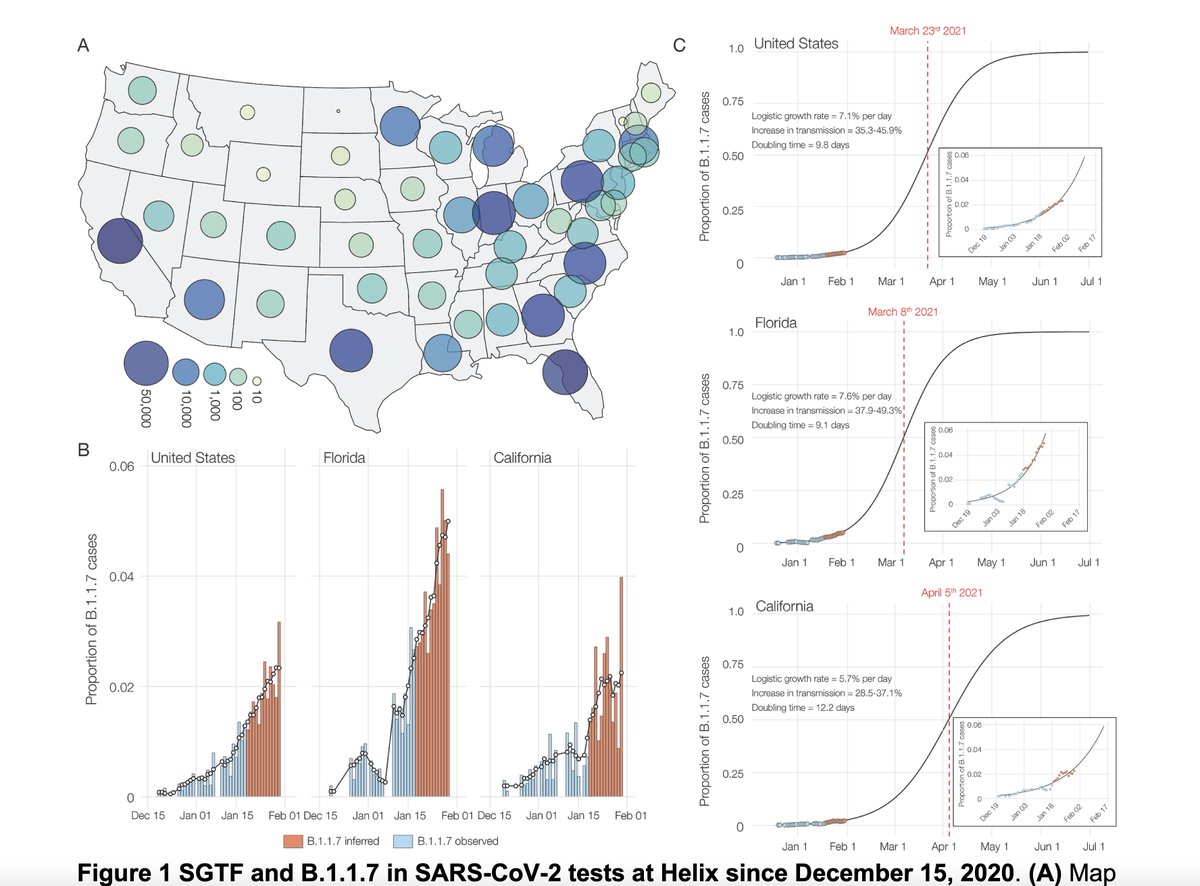
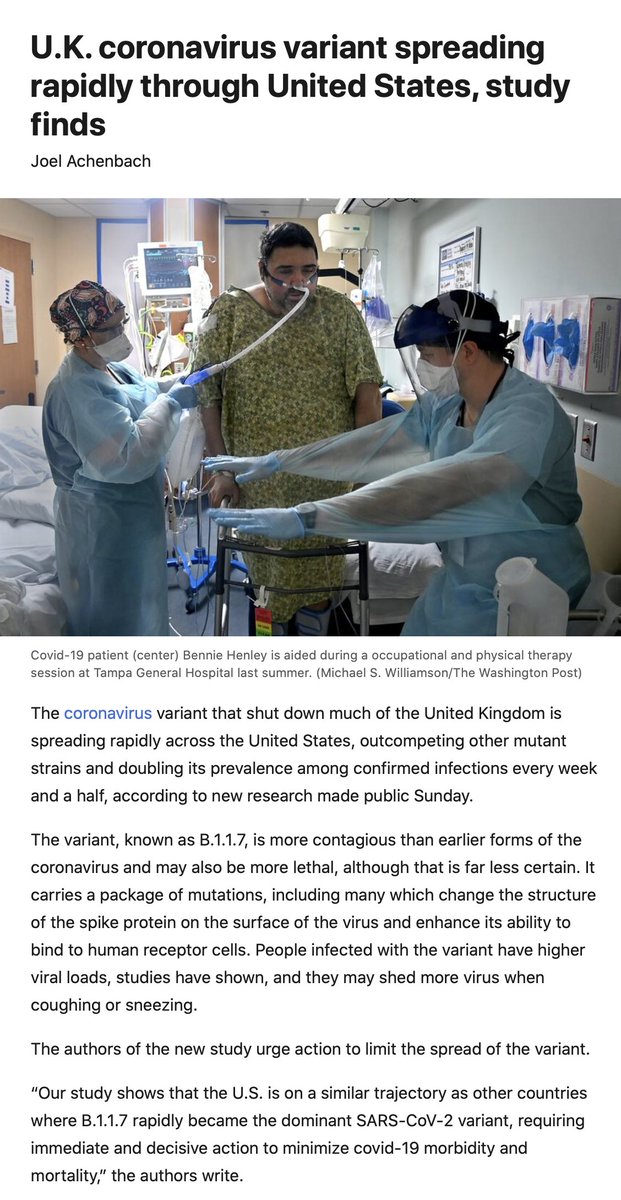
9. Preprint today on B.1.1.7 shows current US spread and forecasting of how the patchwork will play out, calls for immediate and decisive action
medrxiv.org/content/10.110… @K_G_Andersen @my_helix @illumina
and by @JoelAchenbach @PostHealthSci
washingtonpost.com/health/ukvaria…

medrxiv.org/content/10.110… @K_G_Andersen @my_helix @illumina
and by @JoelAchenbach @PostHealthSci
washingtonpost.com/health/ukvaria…
• • •
Missing some Tweet in this thread? You can try to
force a refresh