Back in September, we wrote a @nytopinion on the need to focus on severe infection events prevented by Covid-19 vaccines
nytimes.com/2020/09/22/opi…
Yesterday JNJ presented their Phase 3 data stressing that benefit. I spoke w/ them today & have much more confidence that it's real /1
nytimes.com/2020/09/22/opi…
Yesterday JNJ presented their Phase 3 data stressing that benefit. I spoke w/ them today & have much more confidence that it's real /1

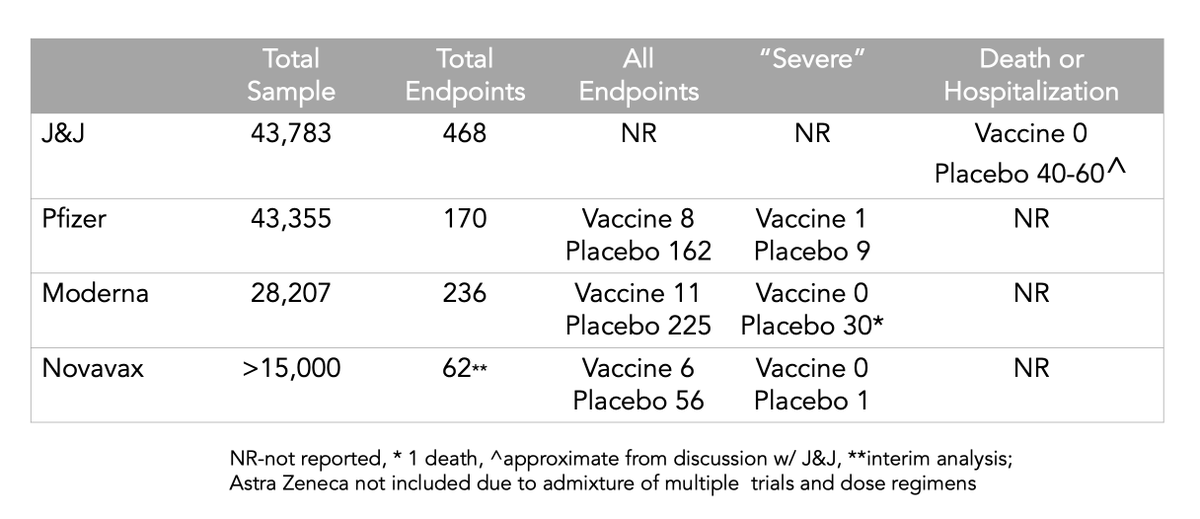
My first question is why didn't they give actual data points and confidence intervals?
Answer: The FDA guided them not to do that. Interesting that the other companies (@Pfizer, @moderna_tx, @Novavax) did provide their data with their press releases /2
Answer: The FDA guided them not to do that. Interesting that the other companies (@Pfizer, @moderna_tx, @Novavax) did provide their data with their press releases /2
Then we reviewed the outstanding finding in their data set.
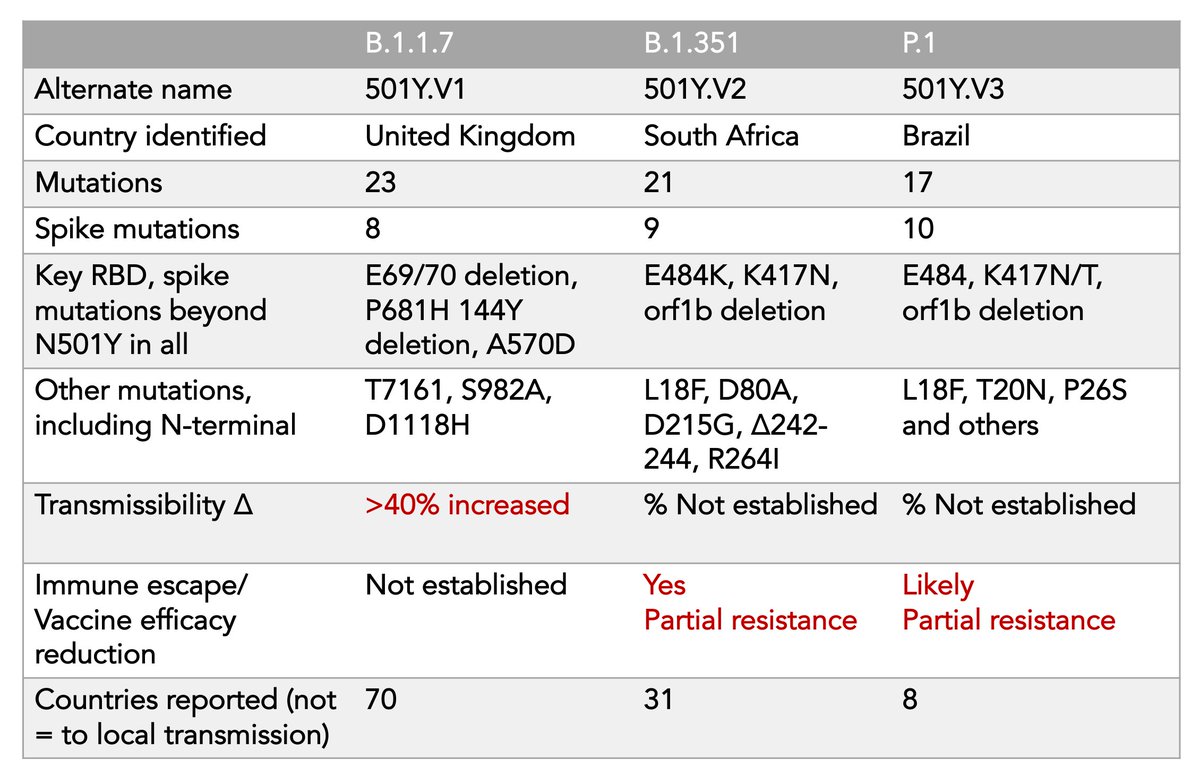
There were approximately 40-60 hospitalizations and deaths in the whole trial. All in the placebo group. Period. Even those occurring in South Africa. That is *great news* /3
There were approximately 40-60 hospitalizations and deaths in the whole trial. All in the placebo group. Period. Even those occurring in South Africa. That is *great news* /3
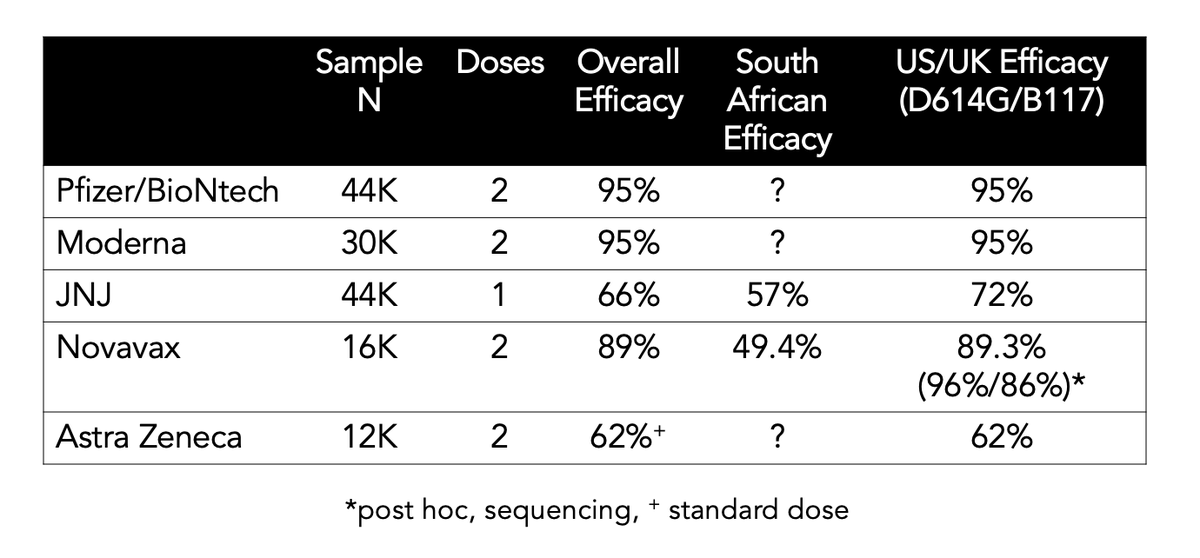
The other thing is the efficacy gap. About ~70% for JNJ (US), and ~90+% for @BioNTech, @moderna_tx, and @Novavax. Review of the definitions of 1° endpoint: there are no substantive differences. It's real; explanation unclear: 1 dose, adenovirus construct, more fit virus, etc /4
Bottom line: Await final data.. But this bolsters my confidence that JNJ vaccine is a major contribution for its ability to suppress the most severe infections, even with B.1.315 (SA variant) 👍 /end
• • •
Missing some Tweet in this thread? You can try to
force a refresh