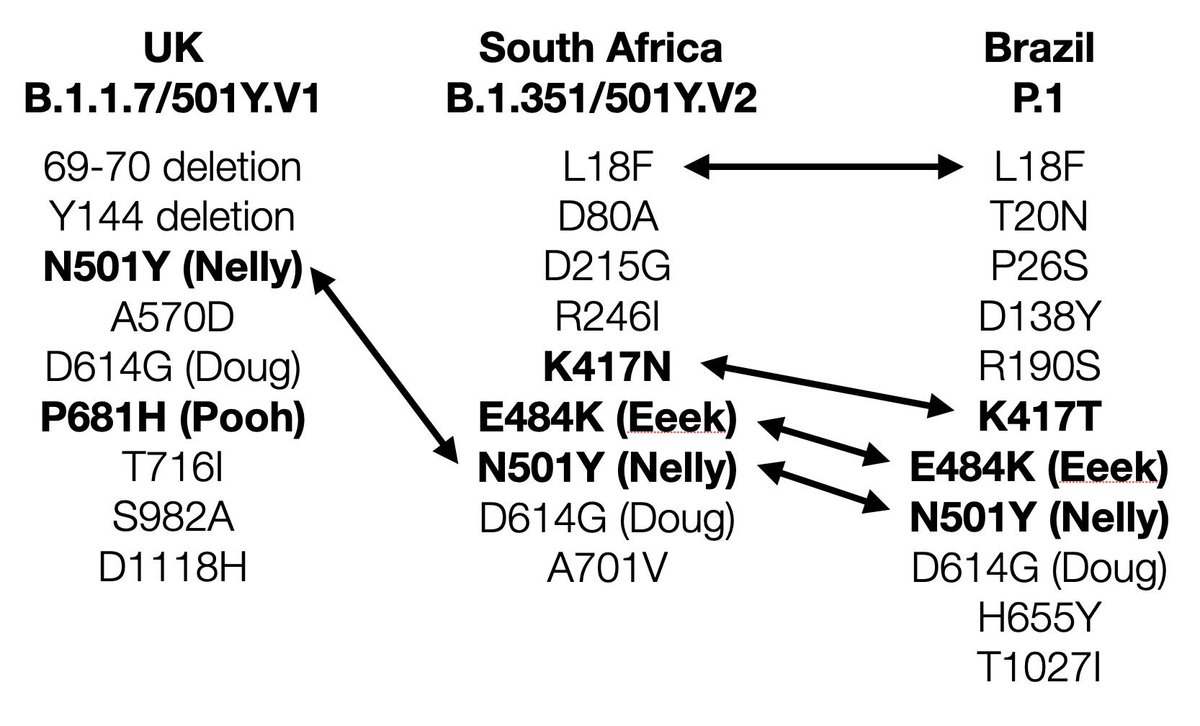
Thread summarising an important presentation on the AstraZeneca trial results for South Africa.
Key points:
➡️ Efficacy initially seemed to be ~75%, but dropped to 22% against SA 🇿🇦 variant.
➡️ Past COVID-19 (original) doesn't protect against reinfection by the SA 🇿🇦 variant.
Key points:
➡️ Efficacy initially seemed to be ~75%, but dropped to 22% against SA 🇿🇦 variant.
➡️ Past COVID-19 (original) doesn't protect against reinfection by the SA 🇿🇦 variant.
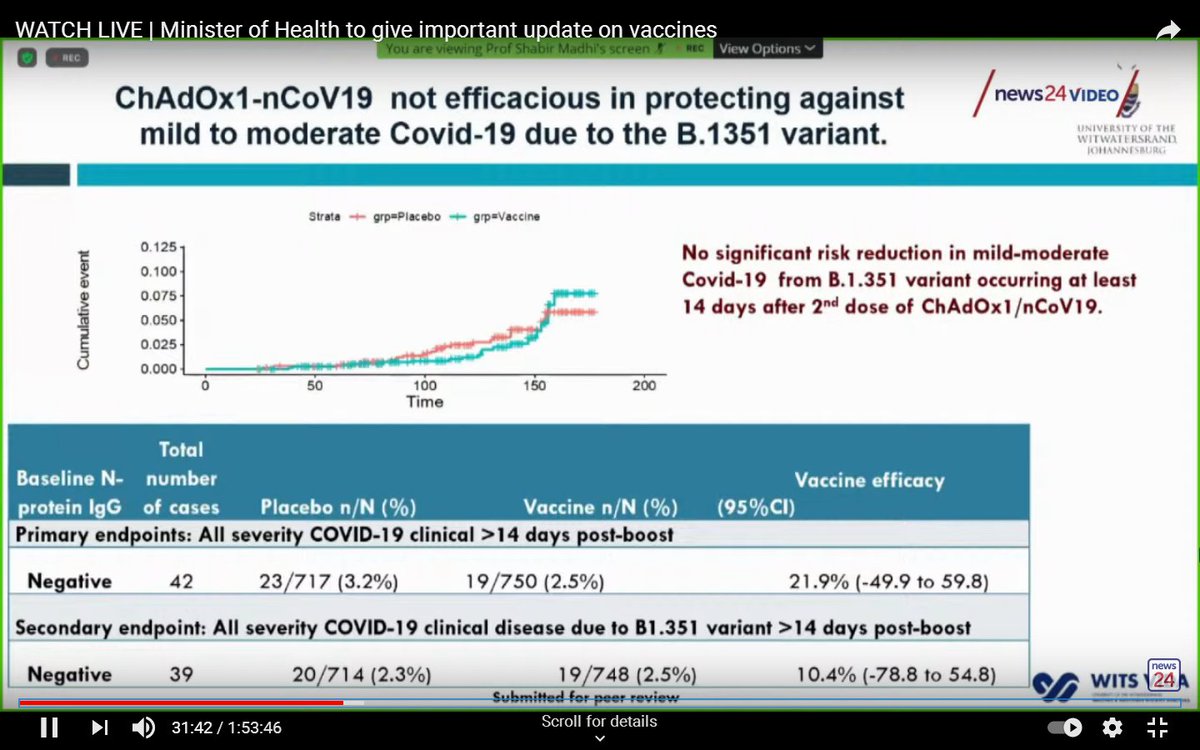
This was a relatively small trial with only 1,749 mostly young, healthy participants.
People were given two doses of the vaccine about 28 days apart.

People were given two doses of the vaccine about 28 days apart.
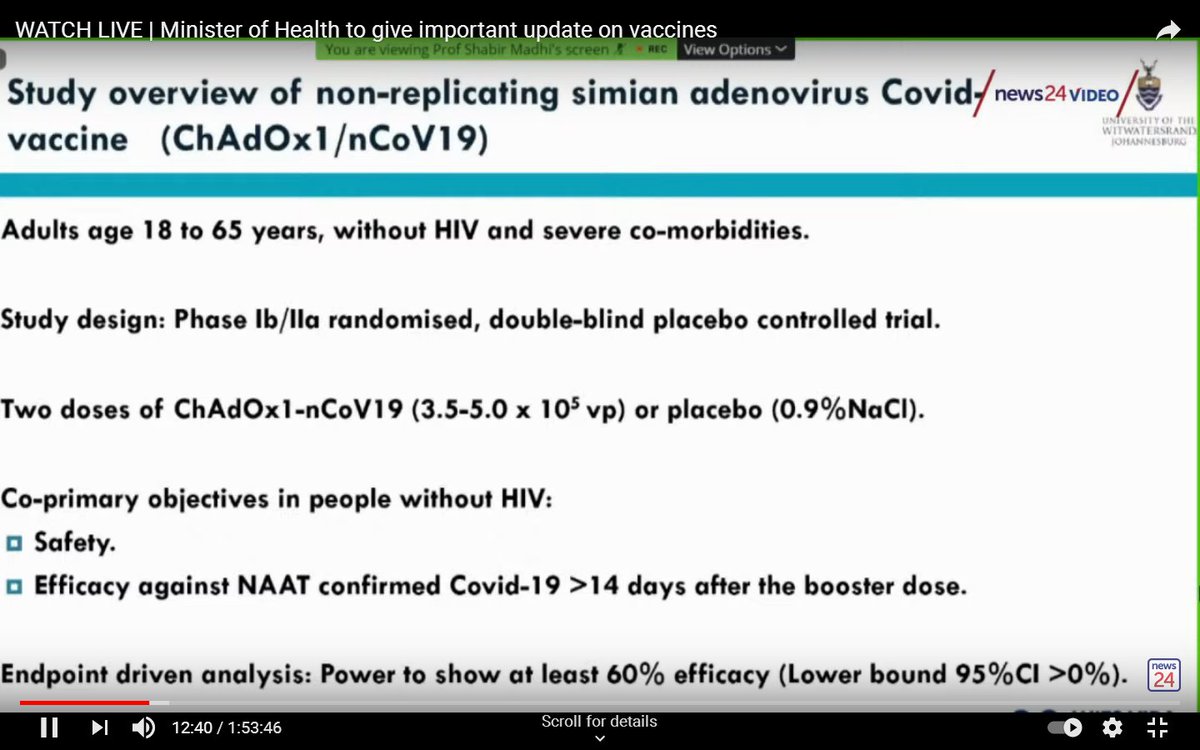
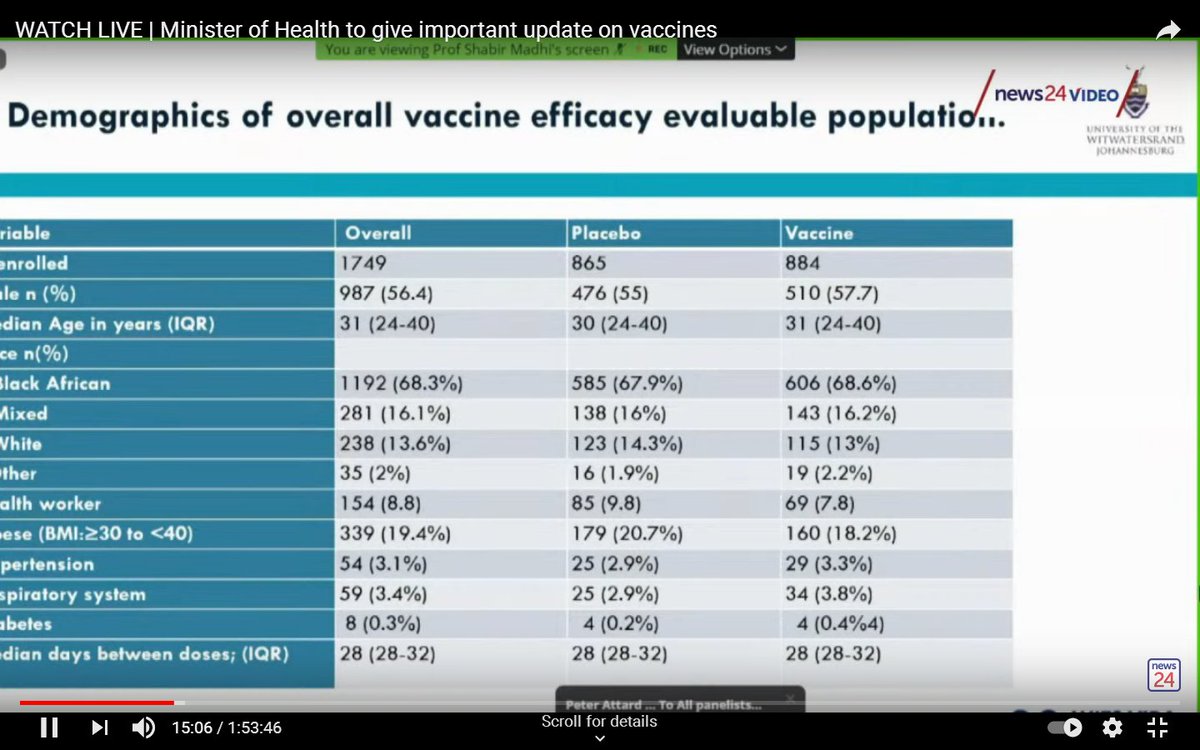
The relatively small number of people in the trial makes it suitable for exploring the efficacy of the Oxford/AstraZeneca vaccine against mild-to-moderate disease only.
The trial lacks statistical power to tell us the efficacy of the vaccine against severe disease.
The trial lacks statistical power to tell us the efficacy of the vaccine against severe disease.
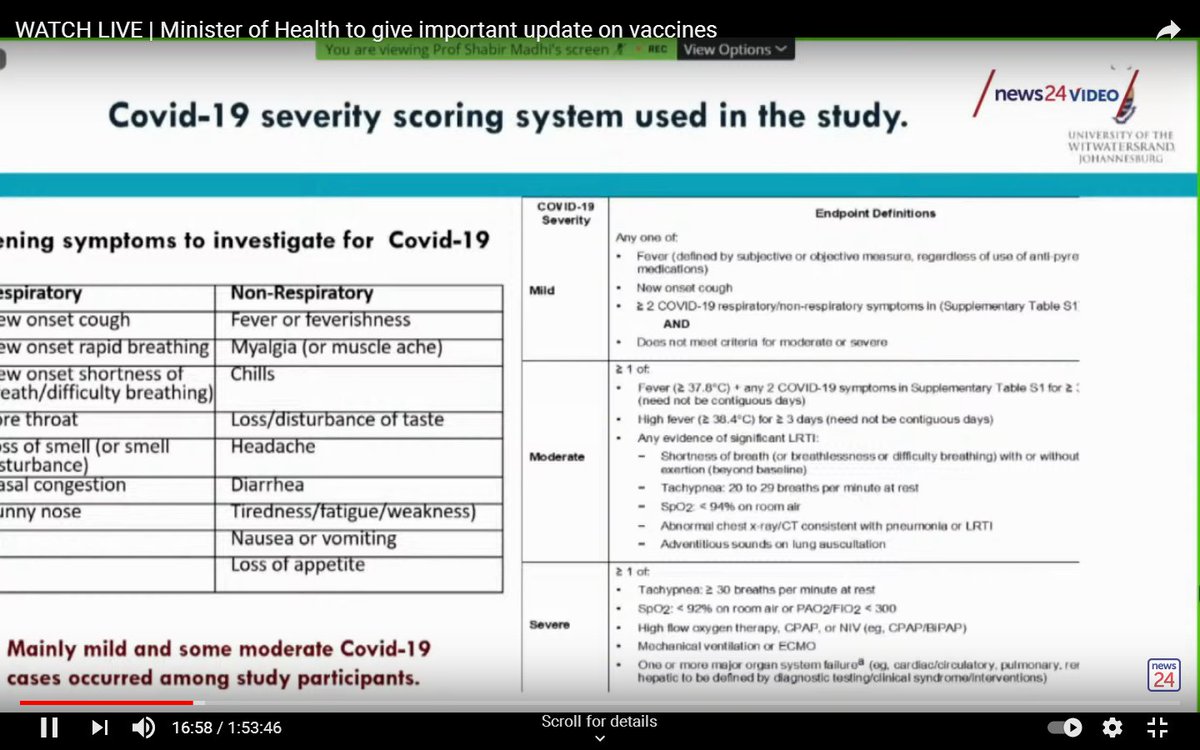
The trial began in June 2020, before the emergence of the South African variant (in September), which is thought to be more transmissible and more virulent.
By the end of the year, the variant accounted for almost all cases in South Africa (orange area of the graph).
By the end of the year, the variant accounted for almost all cases in South Africa (orange area of the graph).
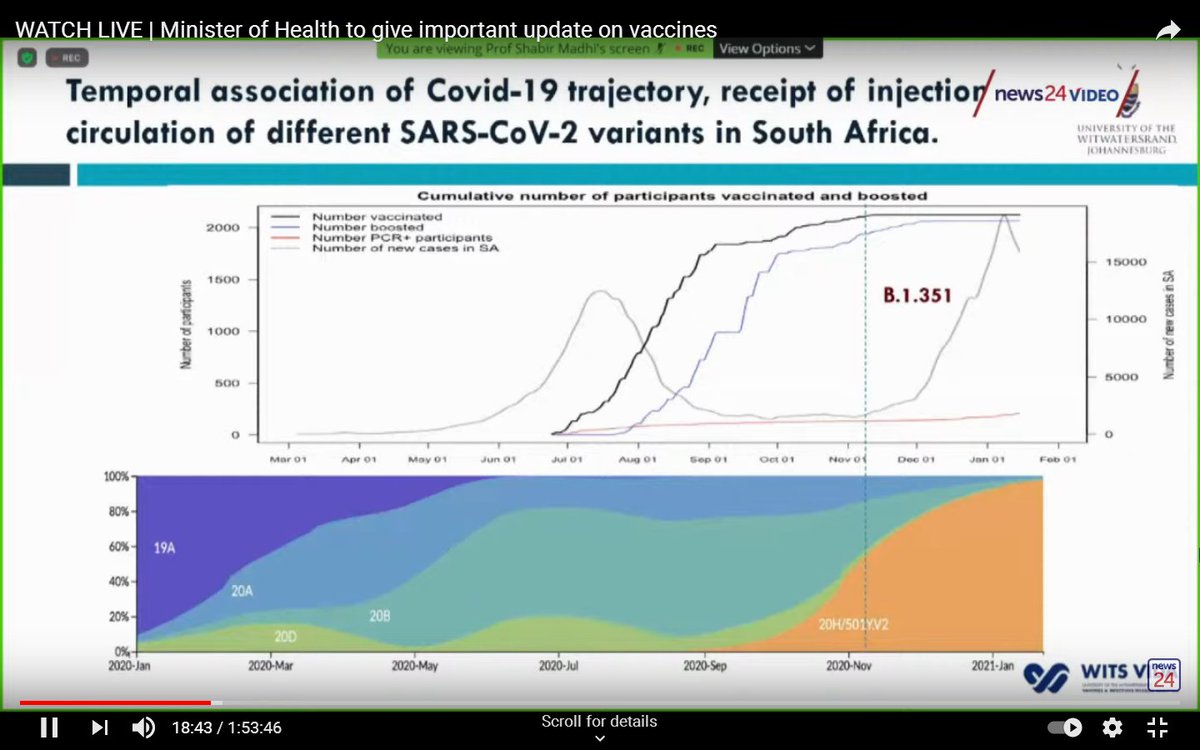
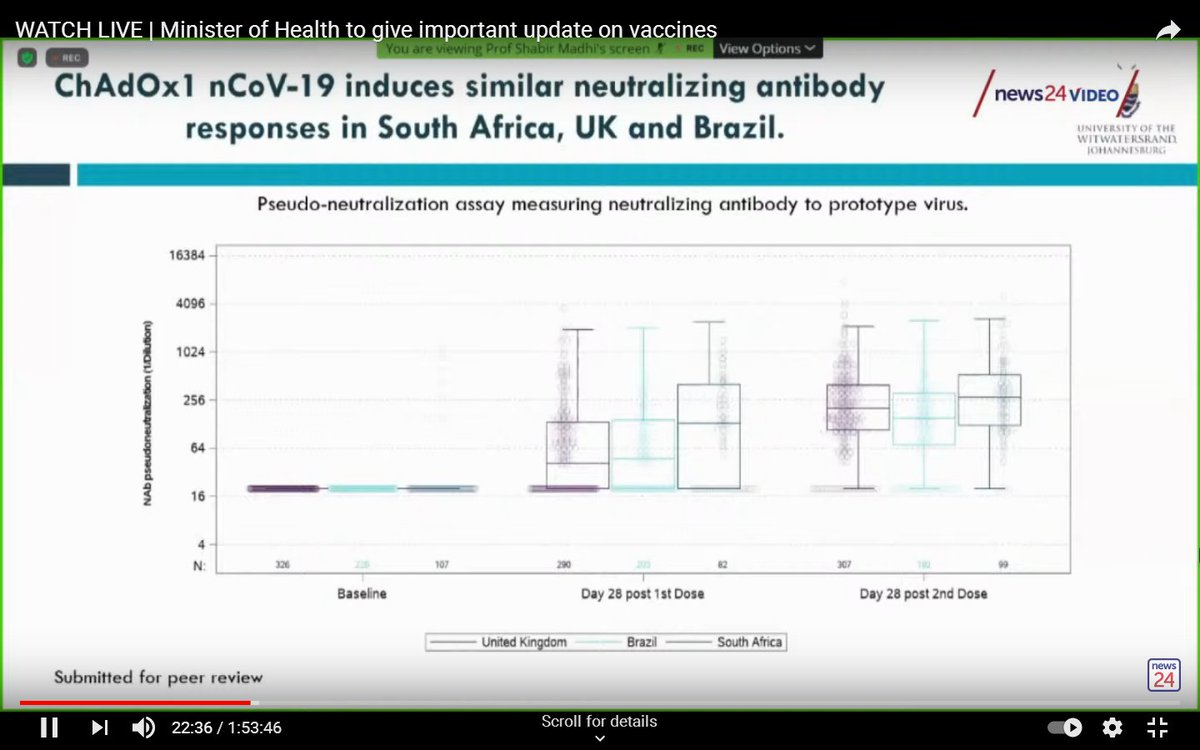
The vaccine produced a good immune response in South African participants, which was comparable to people receiving the vaccine in the UK and Brazil. 
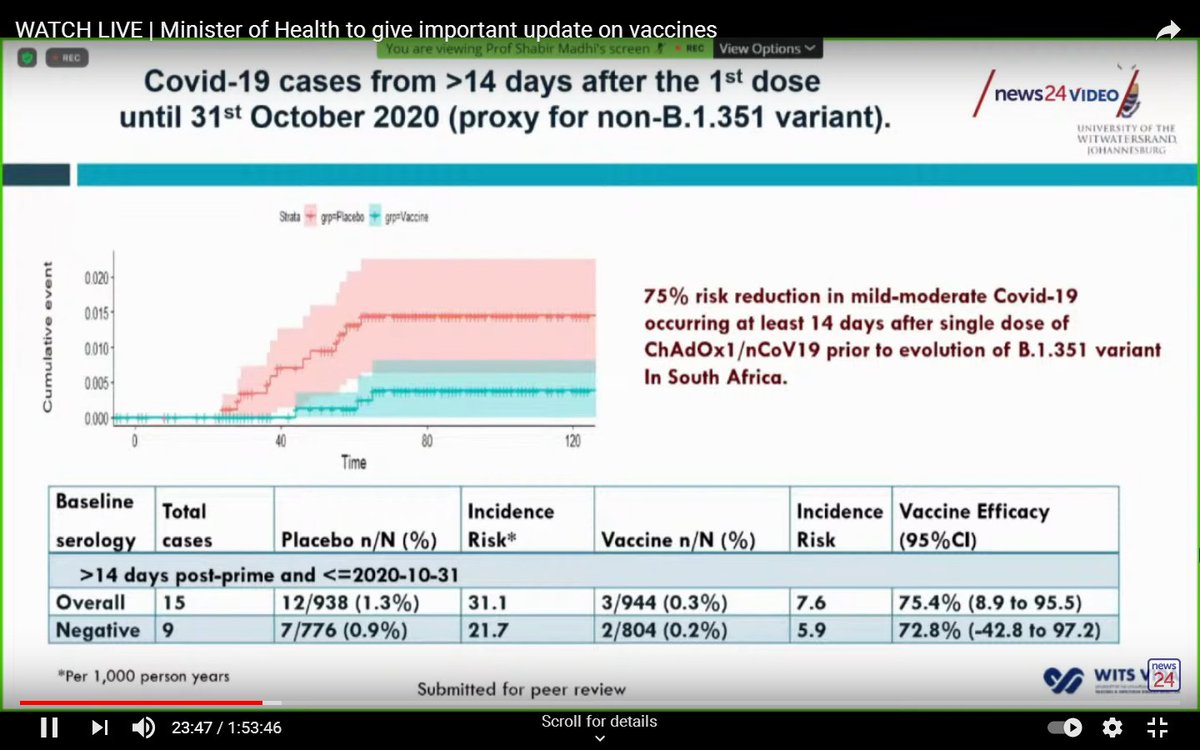
The initial results (until the end of October, before the South African variant was prominent) were promising, and suggested an efficacy of 75% after a single dose. 
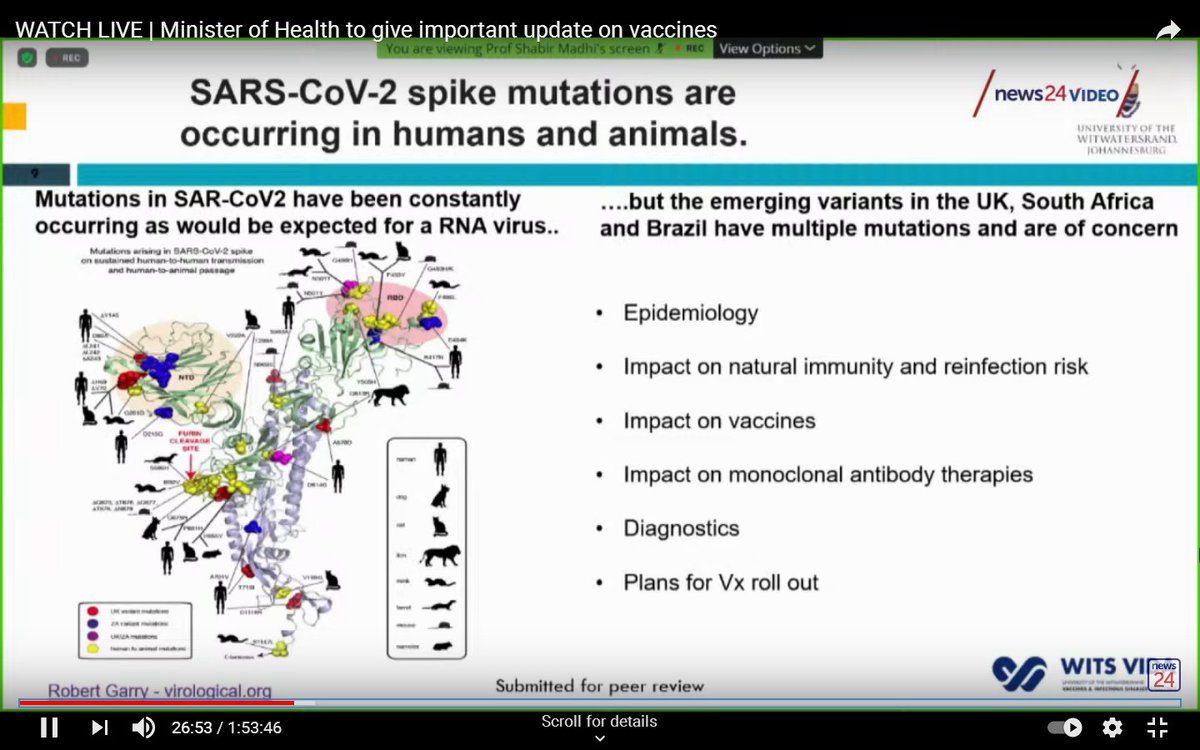
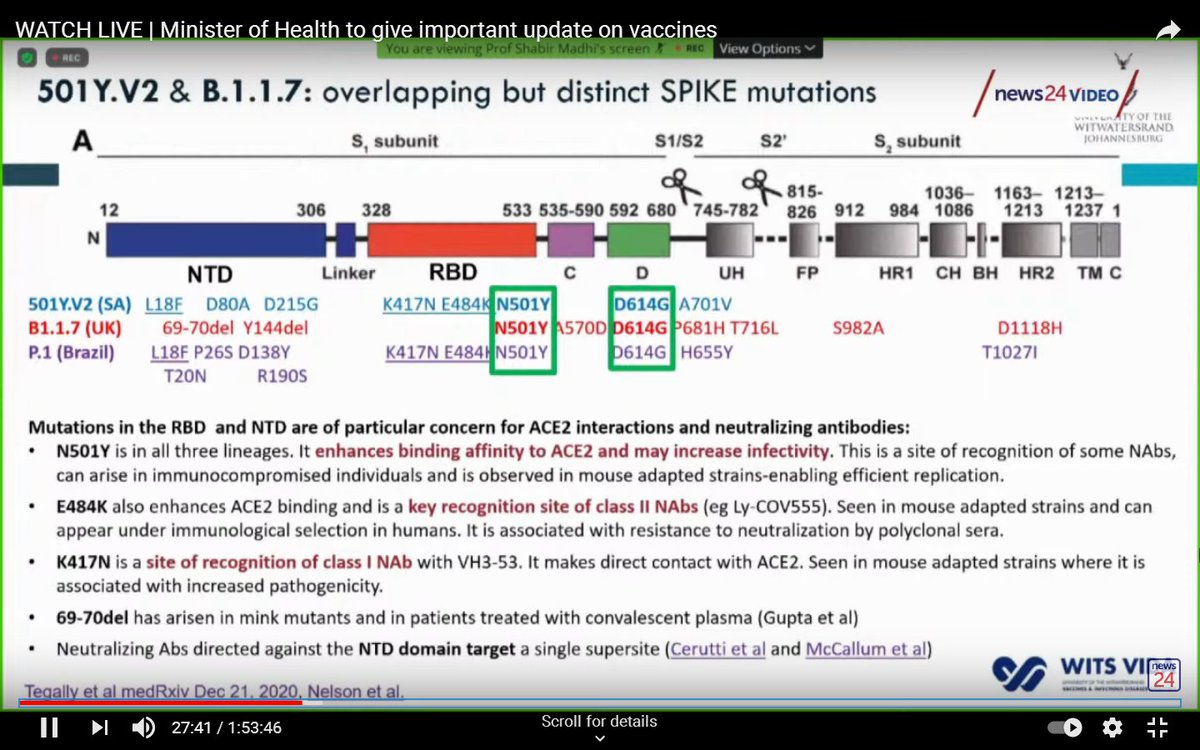
Unfortunately, viruses evolve. It is thought that the very large number of people who had been infected in South Africa may have created pressure that led to the virus mutating. 

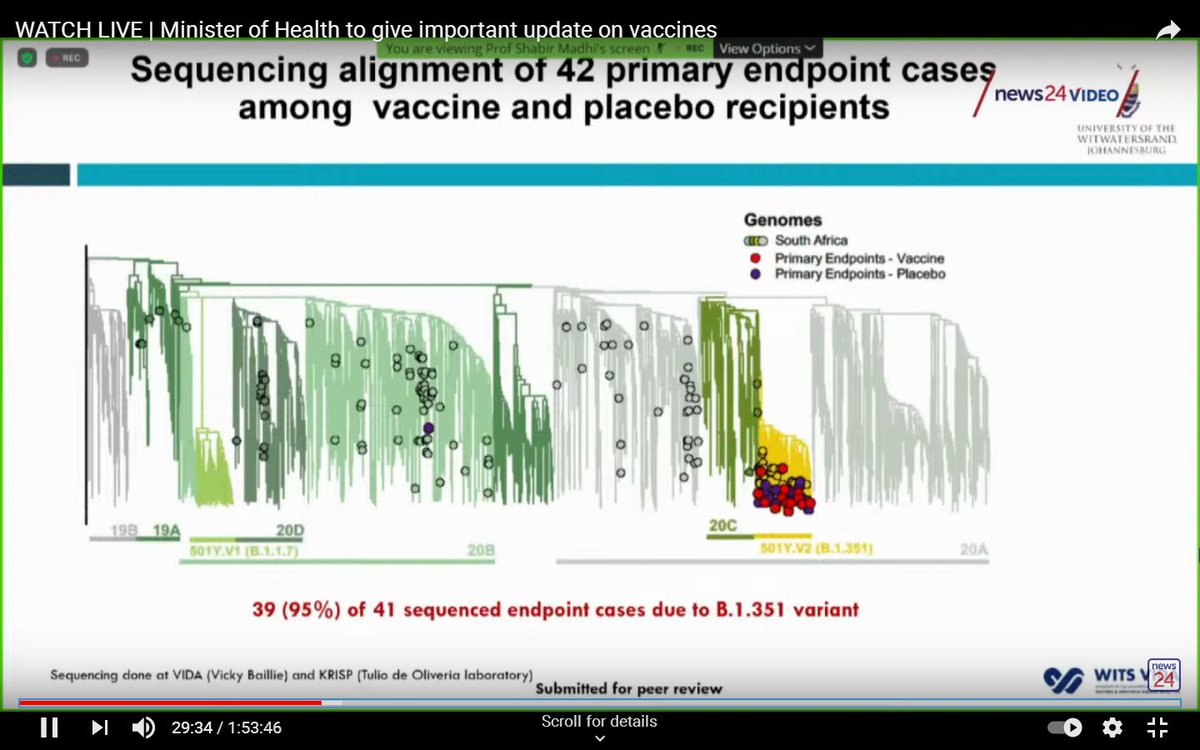
By the end of the trial, 42 cases had been detected.
Sequencing was performed for 41 of these, and 39 (95%) were due to the variant.
Sequencing was performed for 41 of these, and 39 (95%) were due to the variant.
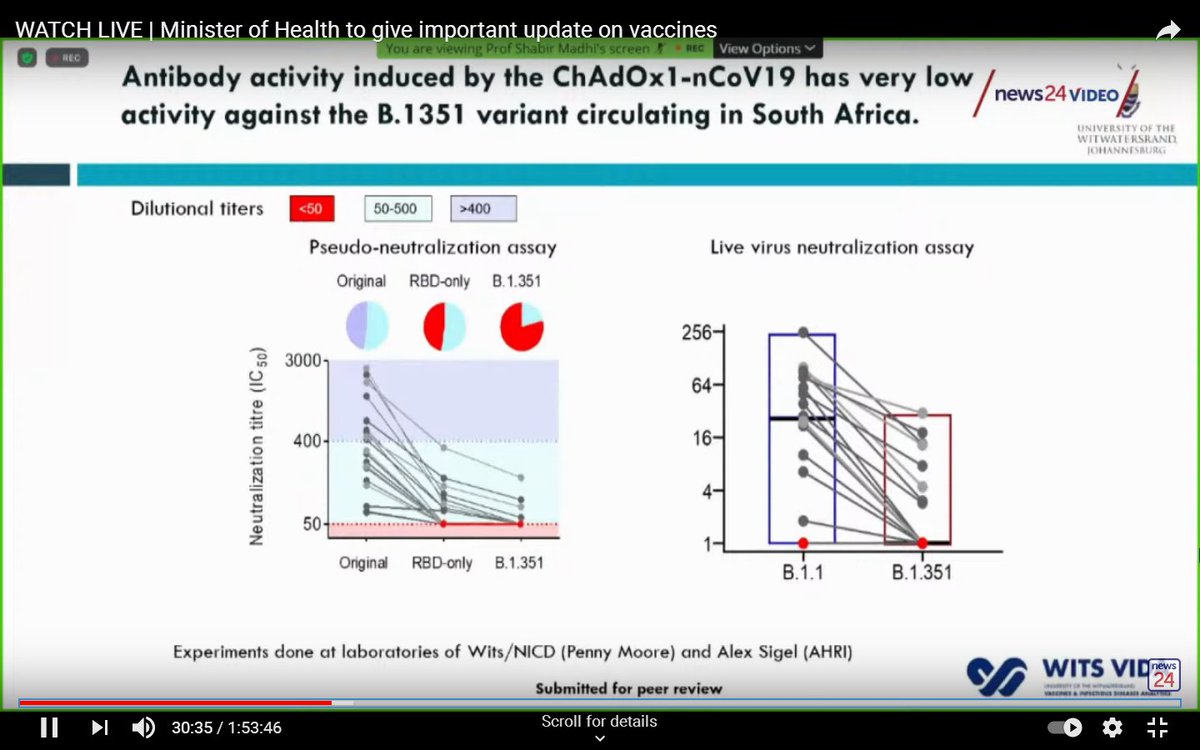
Laboratory experiments showed that the antibodies produced by the vaccine were less effective against the variant. 
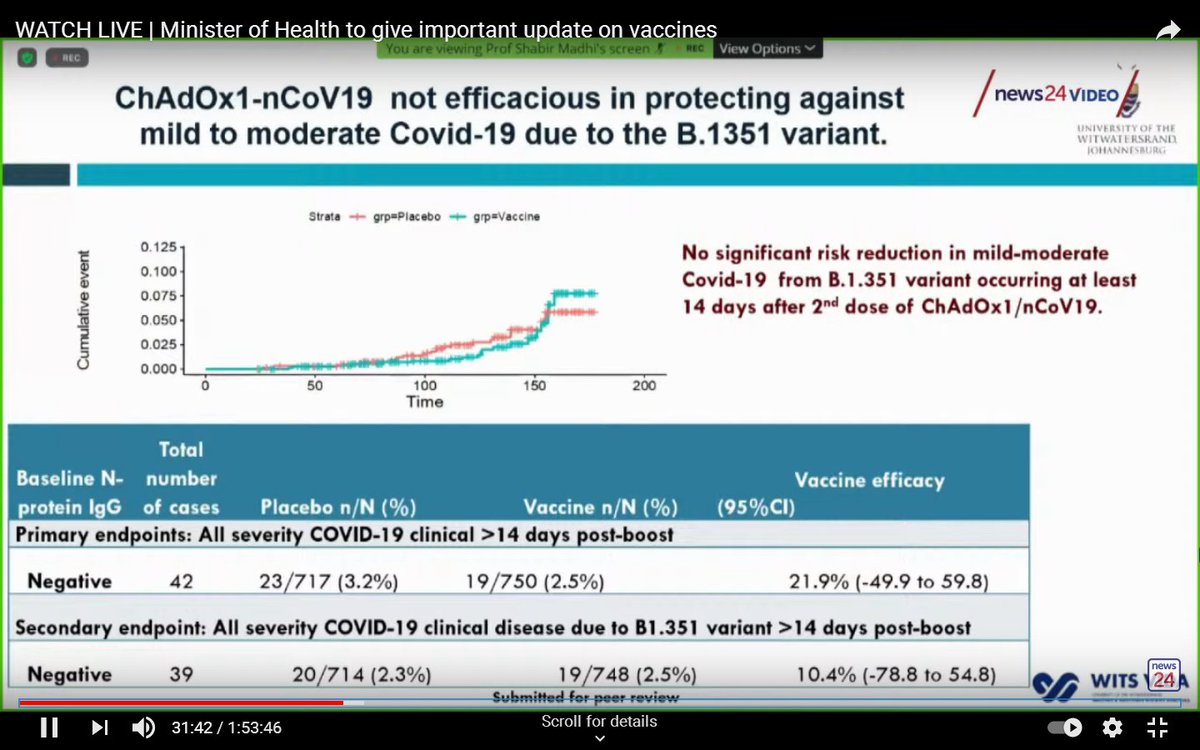
At the end of the trial, efficacy of the Oxford/AstraZeneca vaccine against mild-to-moderate COVID-19 (overwhelmingly caused by the South African variant), was only 22%.
A caveat is that small numbers make this estimate imprecise.
A caveat is that small numbers make this estimate imprecise.
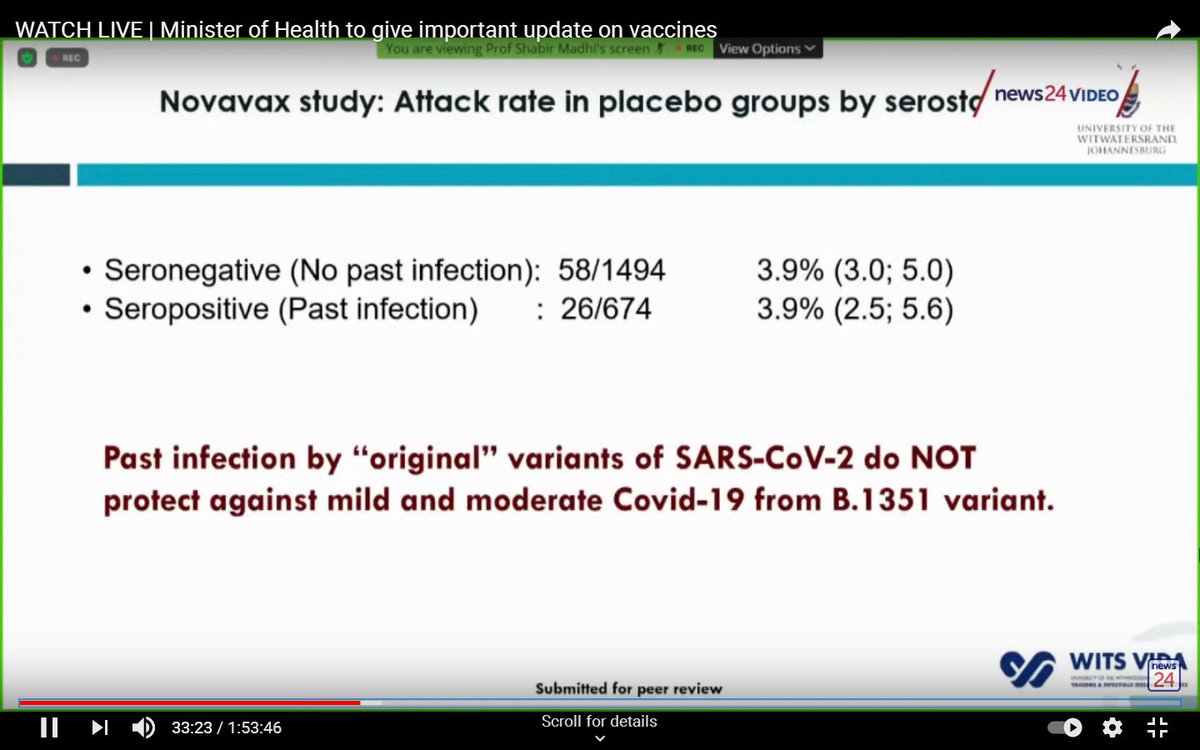
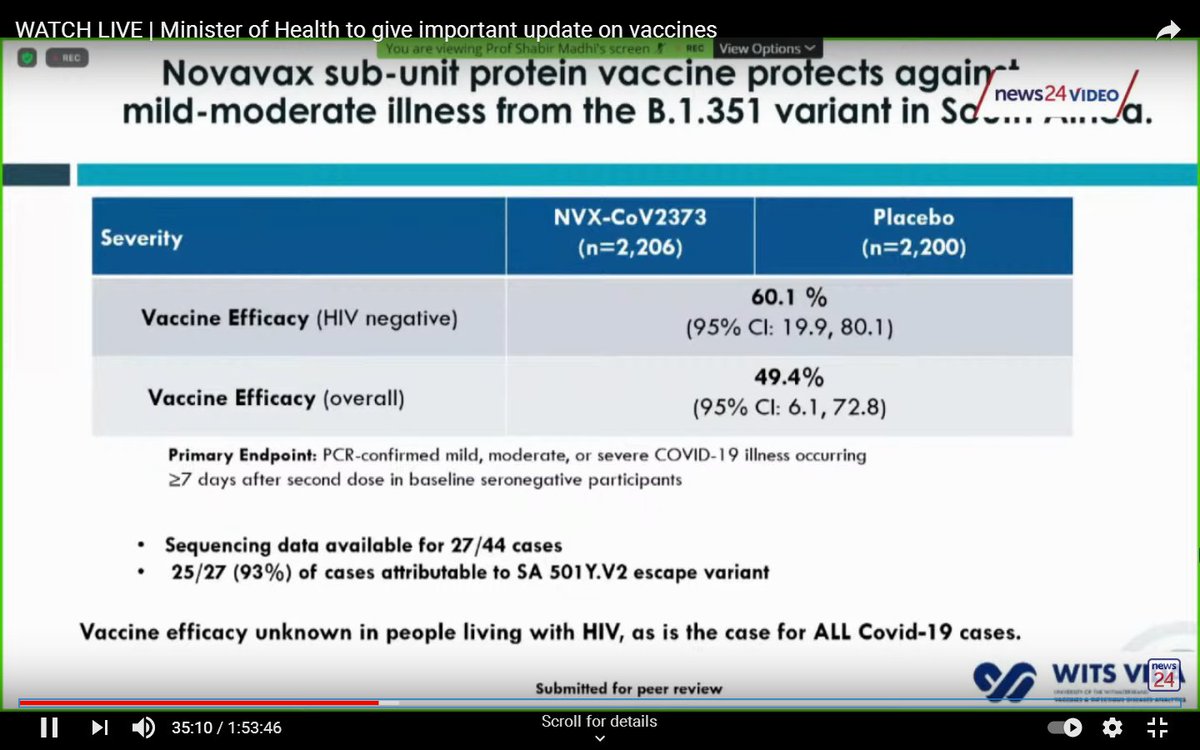
In a separate trial (the Novavax trial) researchers looked at what happened to people in the placebo group, based on whether they had previously been infected with SARS-CoV-2.
They found past infection did not provide immunity against reinfection with the South African variant.
They found past infection did not provide immunity against reinfection with the South African variant.
However, in good news, the Novavax vaccine could still protect against the South African variant, although the efficacy dropped from 95% (against the original strain) to 60% against the South African variant (49% if people living with HIV were included). 
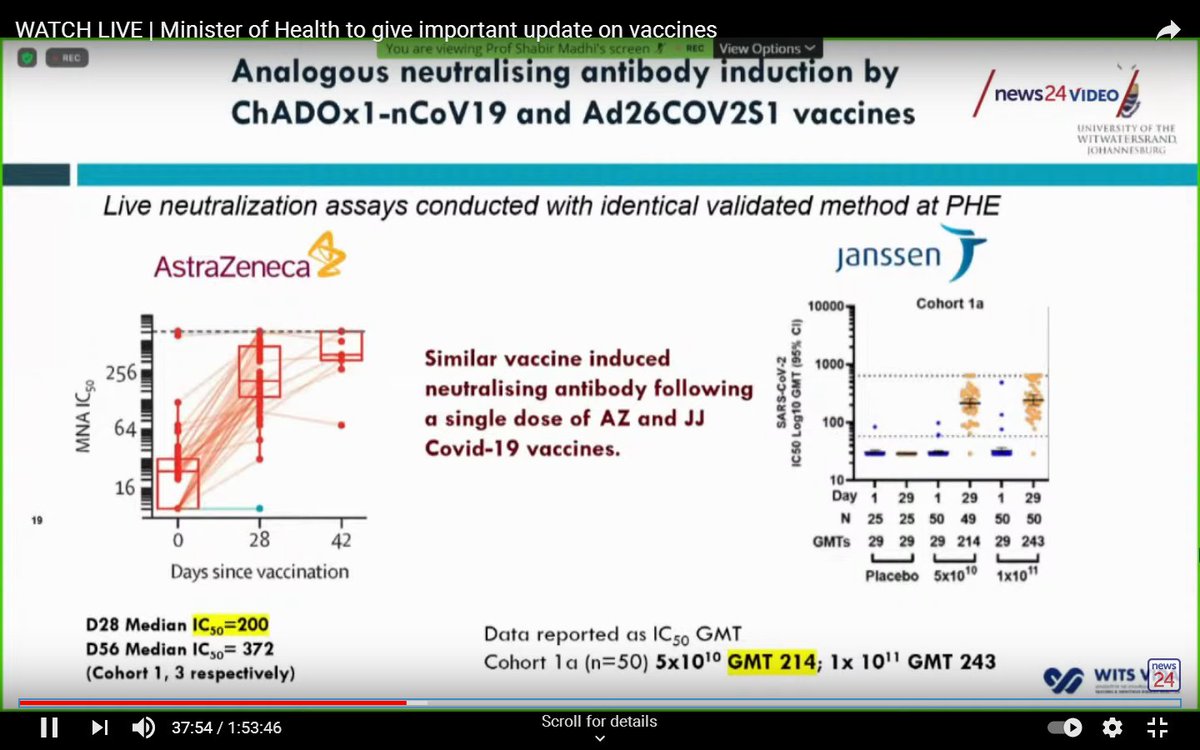
While vaccine efficacy against mild-to-moderate disease has taken a hit with regard to the South African variant, it's expected vaccine efficacy will fare better against severe disease.
This was true for the Johnson & Johnson vaccine.
This was true for the Johnson & Johnson vaccine.

The Johnson & Johnson vaccine uses similar technology to AstraZeneca, and the immune response induced by these two vaccines is similar.
For that reason, the AstraZeneca vaccine may well retain good efficacy against severe disease.
For that reason, the AstraZeneca vaccine may well retain good efficacy against severe disease.
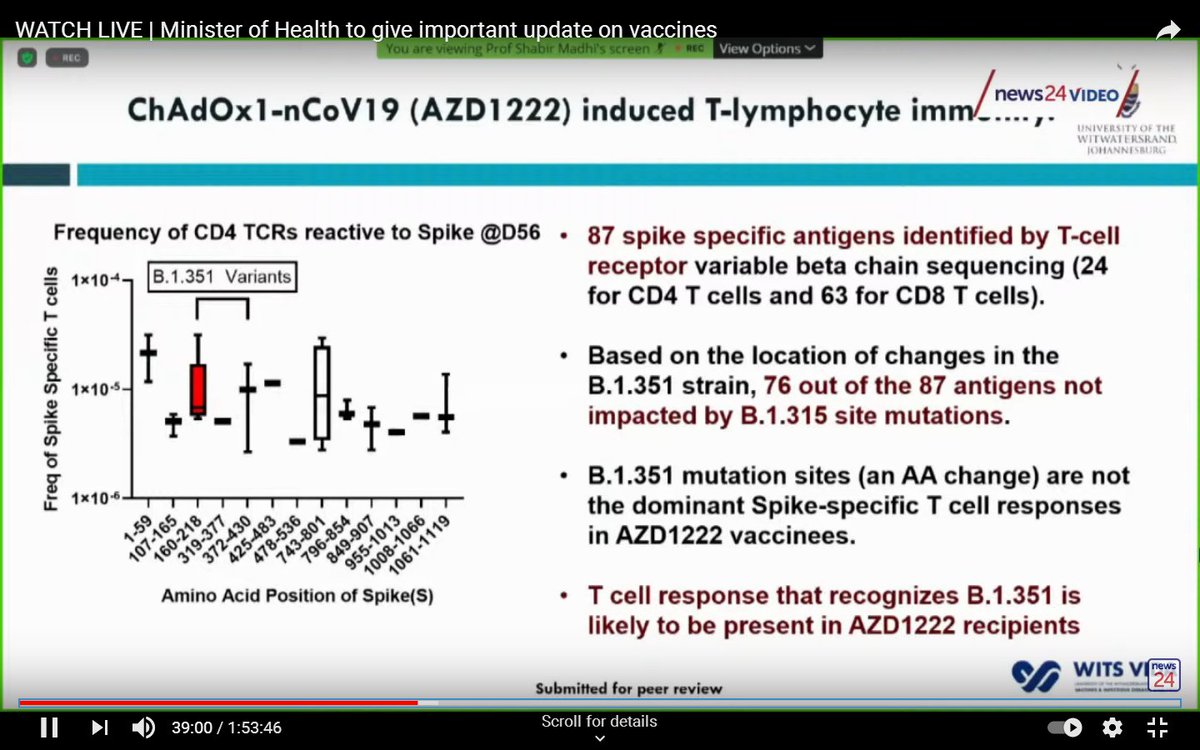
Antibodies aren't the only thing that's important.
T-cells likely play a role in preventing severe disease, and there's evidence to suggest the T cell response is not substantially affected with regard to the South African variant.
T-cells likely play a role in preventing severe disease, and there's evidence to suggest the T cell response is not substantially affected with regard to the South African variant.
In summary, the Oxford/AstraZeneca vaccine does not appear to provide protection against mild-to-moderate disease caused by the South African variant.
However, the Novavax vaccine still retains moderate efficacy.
However, the Novavax vaccine still retains moderate efficacy.

The South African results are a reality check.
Vaccines are still the way out of this pandemic, and high-efficacy vaccines like the Novavax vaccine still work reasonably well.
However, we must reduce transmission so that opportunities for the virus to mutate are reduced.
Vaccines are still the way out of this pandemic, and high-efficacy vaccines like the Novavax vaccine still work reasonably well.
However, we must reduce transmission so that opportunities for the virus to mutate are reduced.

The Oxford/AstraZeneca vaccine remains a useful tool for the time being in badly affected regions where the South African variant is not common (e.g., the United Kingdom).
It will saves lives there.
It will saves lives there.
The full presentation can be found at the link below.
It's well worth watching. There's more data than you can poke a stick at, presented by some great scientists.
It's well worth watching. There's more data than you can poke a stick at, presented by some great scientists.
• • •
Missing some Tweet in this thread? You can try to
force a refresh