Report from Scotland with great news on Covid vaccination reducing hospitalization rates. Not enough data released to be sure about much beyond that though, & number of vaccinated people hospitalized appears to be too small (happily!) to draw much beyond that though...1/2
..Some details about the study hps.scot.nhs.uk/a-to-z-of-topi… BBC says 58 people in hospital who were vaccinated a month before: % by vaccine & for over 80s, but that number seems too small to break it down it by vaccine & age. Overall, ca 90% lower. 🤞 bbc.com/news/health-56… 2/2
...Ah ... preprint tweeted by @jaclark73 - thanks! drive.google.com/file/d/162PJKb… Just hunting out the numbers now... 3/n
..And Public Health England BNT-Pfizer data out too (but I'll start with Scotland)...4/n
https://twitter.com/JustForReading9/status/1363940632016936962
...Scotland: people 80 & over were 1st priority: just over 200,000 people aged 80+ had 1 shot (few had 2). Because BNT-Pfizer was introduced much sooner, only a small proportion of people vaccinated 28 days or longer had Oxford-AstraZeneca...5/n 

..Couldn't see numbers for each vaccine, but total "person years" vaccinated for all age groups 28 days+ are 9,463 for BNT-Pfizer (with 56 people hospitalized), 573 for Oxford-AstraZeneca (with 2 people hospitalized). Here's the sampling of unvaccinated people for comparison..6/n 

...Although the rates of vaccine efficacy for 28 days+ are mostly for BNT-Pfizer vaccine, this figure shows that most people over 70 are getting the Oxford-AstraZeneca vaccine ...7/n 

...Media reports are for the vaccine efficacy rates at their highest points 28 days & later: all time periods from 28 days aren't reported as a single group. At that peak for 80+yr-olds, it was 81% (CI: 65-90): there were 27 events (hospitalizations) (stayed around 80%)...8/n 

...Now to the English report: it's only for BNT-Pfizer vaccine, which makes sense as numbers for Oxford-AstraZeneca are too small for after a month. Top line findings: note not representative for people over 80 as no care home residents assets.publishing.service.gov.uk/government/upl… ...9/n 
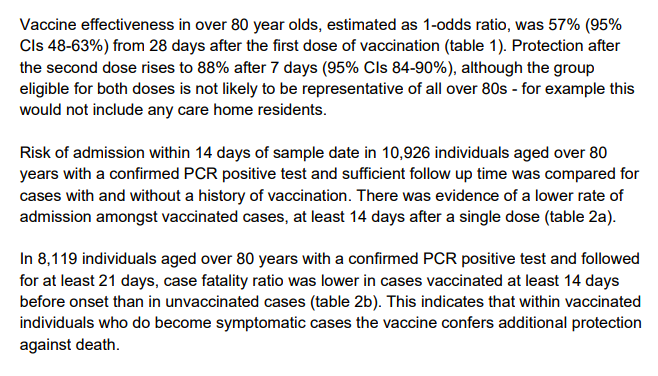
...Limitations include not being able to be sure that people in the comparison group didn't have some immunity from being infected etc...10/n 

...Considering biases in the data, they conclude 3 weeks after a single dose of BNT-Pfizer vaccine, there's more than 50% reduction in symptomatic Covid-19, & for older people when ill, they're half as likely to be hospitalized or die - but that's only 2 weeks after vax ...11/n
...The decline in hospitalizations & deaths in older people is faster in this second lockdown than the first, so they conclude "may therefore be attributable to the high
vaccination coverage achieved in this age group" ...12/n
vaccination coverage achieved in this age group" ...12/n

..Previous pic was over 70s. This is 80+. They report reductions overall are similar to after 1 dose of BNT-Pfizer in Israel & UK study in healthcare workers.
Overall from both these? Too soon for clear picture about Oxford-AstraZeneca, or delaying 2nd dose for either vax. /13
Overall from both these? Too soon for clear picture about Oxford-AstraZeneca, or delaying 2nd dose for either vax. /13

...And we're back to UK results. That UK study in healthcare workers mentioned ⬆️: that preprint is out too ...14/n papers.ssrn.com/sol3/papers.cf… (HT @rajeev_the_king)
...This one's called the SIREN study & it's a prospective study BNT-Pfizer vaccine in healthcare workers in England. For those who were antibody-negative before vaccination, efficacy was 72% (CI: 58-86) 21 days after 1 dose, 86% (CI: 76-97) 21 days after 2nd dose...15/n
...The B.1.1.7 ("UK") variant was dominant during this study. The protocol for the study is here: medrxiv.org/content/10.110… People who joined the study got tested for SARS-Cov-2 fortnightly, antibody testing once a month...16/n
...There were 23,324 healthcare workers in the group reported on: 84% female, 89% white. Data after 2 months. Only reporting on BNT-Pfizer vaccine ...17/n 

...Vaccine efficacy is against infection, B.1.1.7 variant: efficacy 72% (CI: 58-86) 21 days after 1 dose, 86% (CI: 76-97) (middle set below: hazard ratio is 28% - other side of the 72% coin).
So protection against infection by BNT-Pfizer vaccine was high. 🥳 /18
So protection against infection by BNT-Pfizer vaccine was high. 🥳 /18

• • •
Missing some Tweet in this thread? You can try to
force a refresh