I am excited to share our manuscript "Therapeutic Implications of Detecting MAPK-Activating Alterations in Cutaneous and Unknown Primary #Melanomas" published at @CCR_AACR. I think it offers important clinical and translational insights into melanoma. 1/x
clincancerres.aacrjournals.org/content/early/…
clincancerres.aacrjournals.org/content/early/…
All of the genomic and clinical data are available on @cbioportal at cbioportal.org/study/summary?… – thank you to @nikolausschultz Lab, esp @chatila_w + @fjsanchezrivera (bioinformatics/data viz), Arshi Arora (stats), and @sloan_kettering molecular path (all the sequencing) 2/x
This will be a loooong thread for my fellow #melanoma geeks! All other melanoma-curious oncologists and #medtwitter can skip towards the end where I try to highlight some themes for investigating other tumors. 3/x
Most med oncs know CM have genomic alterations serving as “drivers“ of MAP Kinase activity; TCGA classified CM into “BRAF, RAS, NF1, triple-WT.” Good start, but major limitations for modern era of #immunotherapy and targeted tx. 4/x
sciencedirect.com/science/articl…
sciencedirect.com/science/articl…
First, are there unrecognized drivers in the “WT” group or do ~15% truly lack any alterations in this pathway? TCGA used whole exome at relatively shallow mean depth by today’s standards (~87x). Our targeted panel MSK-IMPACT covers 341-468 genes at mean ~700x+ depth. 5/x 

Second, are BRAF, RAS, NF1 the best groups? We know now (thx Neal Rosen lab) there are big differences in BRAF mutations (V600 vs L597/K601 or fusions in Class 2 vs D594 in class 3). How about NRAS Q61 vs G12? What about when multiple drivers overlap? 6/x
nature.com/articles/natur…
nature.com/articles/natur…
Third, and perhaps most importantly, who cares about identifying driver mutations beyond BRAF V600 when we are living in the immunotherapy era? I'm a busy medical oncologist...help me help my patients! 7/x
To answer these ?s, we merged high depth sequencing, detailed clinical annotation, and treatment outcome data. In the process, we learned a lot of things directly relevant to clinical care and I’ll try to highlight a few of these. 8/x
Using 696 unique CMs seq as standard care (>2x size of TCGA set), we can start to answer the first question about “triple wild-type” CMs using 28 genes in RTK-RAS-MAPK pathway. We considered only oncogenic alterations per @OncoKB (hi @CDebyaniPhD!) to limit noise/passengers. 9/x
We included unknown primary melanomas because we showed that unknown primary melanomas look essentially identical to known cutaneous primary melanomas by MAPK driver, median TMB, etc. Wild to think that our bodies reject cutaneous melanomas, but they do… 10/x 

So, first answer: 96% of CMs have a known somatic RTK-RAS-MAPK driver using 28 genes. Investigation is ongoing in the rare “pan-WT” melanomas. So if you sequence a melanoma and there is no known driver, send a new sample or use a new platform! 11/x 

Because many CMs have multiple mutations, it is hard to know how to handle these clinically (e.g. BRAF D594N, NF1 nonsense, NRAS G12D = ???). We used a combination of co-alteration analysis and median TMB to make a mutually exclusive, hierarchical group of 9 groups. 12/x
Some alterations often arise as the ‘sole drivers’ of the MAPK pathway (BRAF V600E or K, NRAS Q61) while other alterations are almost always co-altered (NF1, a bevy of rare RTKs like HER2, KIT, etc). Will return to this later as I think it’s therapeutically relevant. 13/x 

For BRAF, we extend prior Rosen Lab findings that BRAF D594, other Class 3s are almost always co-altered with something else, e.g. NF1, KIT, etc; BRAF V600E or K rarely. So these are entirely distinct melanomas, signaling-wise. 14/x 

Similarly, in the RAS group, NRAS Q61 is distinct from other alterations (e.g. KRAS or NRAS G12). It has a lower median TMB, arises in younger folks, and is less often co-altered (same figure). So we separated those. 15/x 

Was this because these co-alterations arose in different clones captured by high seq depth? Nope! We analyzed clonality (using FACETS) in a suitable subset to show that vast majority of these alterations are clonal and not subclonal. This arose in peer review; v. grateful. 16/x 

OK so! Let's use MAPK drivers to show some cool relationships you can use in clinical care. Men are more likely to get head/neck primaries that are enriched for NF1 and high TMB; BRAF V600 less likely there. As a guy with rapidly thinning hair, I feel this intensely. 17/x 

Perhaps unsurprisingly, median TMB in #melanoma increases proportionally with age. I can’t help but think this is a nice fact for geniuses like @AshaniTW @whitefishlab and others interested in aging TME, metabolism of specific melanomas. 18/x 

OK so back to the third and biggest question: who cares about drivers in the era of PD-1 blockade? Nine driver groups is too hard to remember in clinic! Do MAPK drivers offer info independent of TMB? Wait, is TMB even important here at all? 19/x
We used N=322 patients to investigate frontline PD-1 blockade (prior advanced tx excluded) with ipi (combo, N=141) or alone (mono, N=181).
Driver class matters! Time to tx failure of PD-1 mono but not combo varies by simplified 4 groups (BRAF V600, NF1, NRAS Q61, Other). 20/x
Driver class matters! Time to tx failure of PD-1 mono but not combo varies by simplified 4 groups (BRAF V600, NF1, NRAS Q61, Other). 20/x

As a side note, I think we definitively show TMB is associated with outcomes for both PD-1 mono and combo. Time to treatment failure and OS vary by TMB. Prior studies suggesting otherwise were smaller and included folks that had progressed on prior ipi etc. 21/x 

On multivariable analysis, we were surprised to see 4-Driver Class was independently associated with TTF of PD-1 monotherapy even after incorporating TMB, Stage, ECOG performance status. Cool!
Note - this was another request from, and win for, peer review! 22/x
Note - this was another request from, and win for, peer review! 22/x

What if you don’t have any access to fancy TMB/genomics? If all you knew was the primary site of melanoma, we show that TTF of PD-1 monotherapy is best for the head/neck primaries rather than other sites or unknown primaries. Combo had no relation. Kinda cool, right? 23/x 

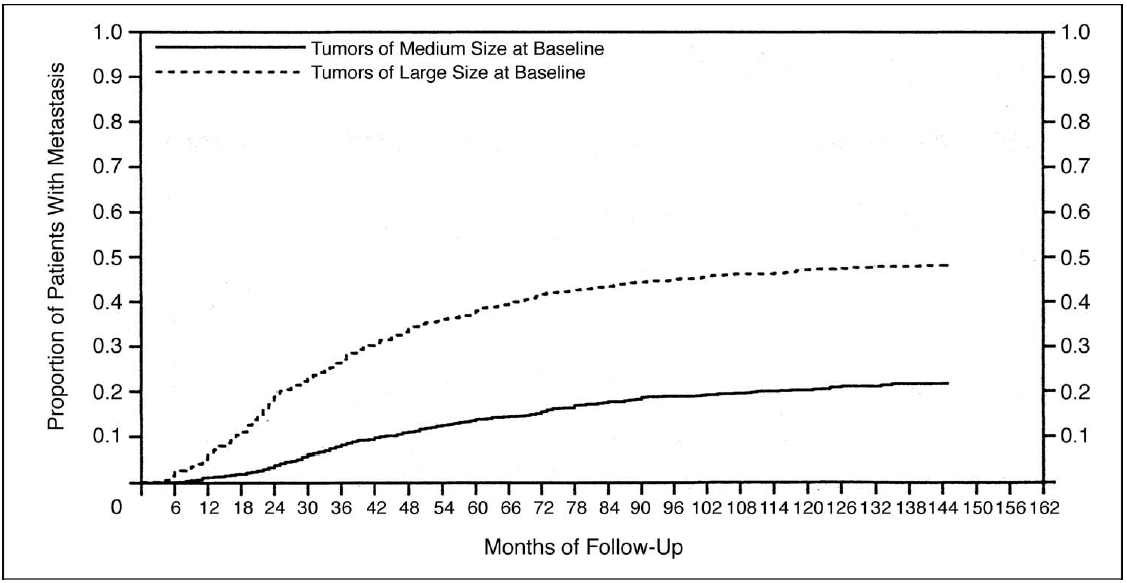
Putting exuberance aside, how often does knowing the MAPK driver at a tertiary cancer center actually matter for those who progress on PD-1 blockade? One has to sequence ~21 BRAF V600 WT tumors to find 1 pt who got >6 months of benefit from driver-selected tx. 24/x
Some folks benefitted a ton, mainly w/ fusions (BRAF, NTRK, ROS1) getting TKIs. Likely not coincidental that these are often “sole drivers” of MAPK, not co-altered. “Low hanging fruit” of TKI mono-tx in melanoma may be exhausted but this offers roadmap for combo targets. 25/x 

This work has directly influenced my #melanoma practice. I (and now you!) can infer a lot from knowing someone's tumor is NF1 or NRAS G12 mutant vs NRAS Q61; from a scalp primary vs trunk vs unknown primary; or M1b stage. 26/x
Please don’t be a fatalist! Send multigene sequencing in someone with BRAF V600 wild-type melanoma progressing on therapy. # needed to sequence is 21 now, but should improve. E.g. if RAF dimers work in NRAS mut, SHP2 in NF1, etc. (Fonz voice: "aaaayyyyyy!") 27/x
Major limitations in this paper
All retrospective, no PD-L1, infiltrates, or transcriptomics. PD-1 mono vs combo was not randomized; we chose who needed which therapy. F/U median was ~2.5-3.5 years, need more. Didn’t correct TMB by MAF/clonality (sorry @ShainLab!). 28/x
All retrospective, no PD-L1, infiltrates, or transcriptomics. PD-1 mono vs combo was not randomized; we chose who needed which therapy. F/U median was ~2.5-3.5 years, need more. Didn’t correct TMB by MAF/clonality (sorry @ShainLab!). 28/x
This work suggests RAS mutant biology melanoma (NF1/NRAS mut) are a major area of unmet need in #melanoma (just like every malignancy, sigh). And to bench folks: use these co-alterations to make better preclinical models! Some of them are whack. 29/x
Some questions for other histologies:
I wonder if these relationships for “sole vs multiple drivers,” TMB, PD-1 outcomes vary in other histologies like NSCLC, bladder, etc.
CC: @OncoAlert colleagues, @PatelOncology @PGrivasMDPhD
30/x
I wonder if these relationships for “sole vs multiple drivers,” TMB, PD-1 outcomes vary in other histologies like NSCLC, bladder, etc.
CC: @OncoAlert colleagues, @PatelOncology @PGrivasMDPhD
30/x
With such a modest median TMB for BRAF V600E melanomas, I wonder why they even respond to PD-1 as well as they do…strikes me that in NSCLC, this is the only “targetable” driver subset with a decent ORR to checkpoint inhibitors.
31/x
31/x
I suspect this has a lot to do with metabolism but I’m not smart enough to know how, so I defer to colleagues like @merghout @RZappasodi @wolchokj @SantoshVardhana to investigate! 32/x
Oh and thank you also to so many other folks e.g. @DSolit, @DrBetofMDPhD @MPostow who didn’t force me to break up this monstrosity into multiple manuscripts.😊
Onward and upward! Kudos if you read to the end...
33/33 Fin
Onward and upward! Kudos if you read to the end...
33/33 Fin
• • •
Missing some Tweet in this thread? You can try to
force a refresh