Is vaccination protection against severe disease higher than against mild disease? Key Q for new variants which may show lower effectiveness against mild cases.
Thread
tl;dr Data do not show higher efficacy against severe disease, but not clearly lower either. It matters.
Thread
tl;dr Data do not show higher efficacy against severe disease, but not clearly lower either. It matters.
https://twitter.com/biosbenk/status/1373955923396411393
Background
COVID-19 has reshaped our society for the last year b/c of the 10-20x higher hospitalization rate & fatality compared to the flu.
However, vaccine efficacy trials for COVID-19 have included all "symptomatic infection" which is mostly mild cases. (even for J&J)
COVID-19 has reshaped our society for the last year b/c of the 10-20x higher hospitalization rate & fatality compared to the flu.
However, vaccine efficacy trials for COVID-19 have included all "symptomatic infection" which is mostly mild cases. (even for J&J)
This was done b/c mild cases occur more frequently; efficacy could be measured more quickly if mild cases were included. Some (@EricTopol) raised concerns about this design b/c they were worried that vaccines might *only* prevent mild cases & not severe.
nytimes.com/2020/09/22/opi…
nytimes.com/2020/09/22/opi…
Others argued the converse: that protection against severe disease will be higher than protection against mild disease. Very nice article by @nataliexdean w/ some examples of this (although most differences shown were not significant, & just trends).
blogs.bmj.com/bmj/2021/03/05…
blogs.bmj.com/bmj/2021/03/05…
Here's thread from @profshanecrotty which assumes higher protection against more severe disease (& raises additional Q of how protection from infection varies w/ protection from disease):
https://twitter.com/profshanecrotty/status/1367559003534811136
Why does this matter now? Biggest reason to me is that some vaccines have very low efficacy against mild disease w/ new virus variants (e.g. AZ in S Africa:
https://twitter.com/DiseaseEcology/status/1371952030424518656). Should we assume, as WHO did, that they'll still protect against severe disease?
WHO recommended AZ vaccine still be used in Africa, despite showing essentially no efficacy against mostly mild cases of B.1.351 in S Africa (
wsj.com/articles/who-r…
https://twitter.com/DiseaseEcology/status/1371952030424518656) on the grounds that vaccine would still protect against severe disease from B.1.351.
wsj.com/articles/who-r…
While I know of no data to support WHO's recommendation to use AZ vaccine in Africa despite little protection against mild cases in S Africa, it's worth asking, more broadly, is protection against severe disease higher than protection against mild disease?
One might think tons of data supports this given all the press releases reporting "100% protection against hospitalizations, severe disease & death". But # events usually tiny (e.g. 5,0) so 100% really means 48%-100%. See @hildabast for more: theatlantic.com/health/archive…
Here's the data we have. There are efficacy data for 3 vaccines from RCTs, and 1 effectiveness study on Pfizer vaccine in Israel with data on efficacy or effectiveness against different disease severities. What do studies show?
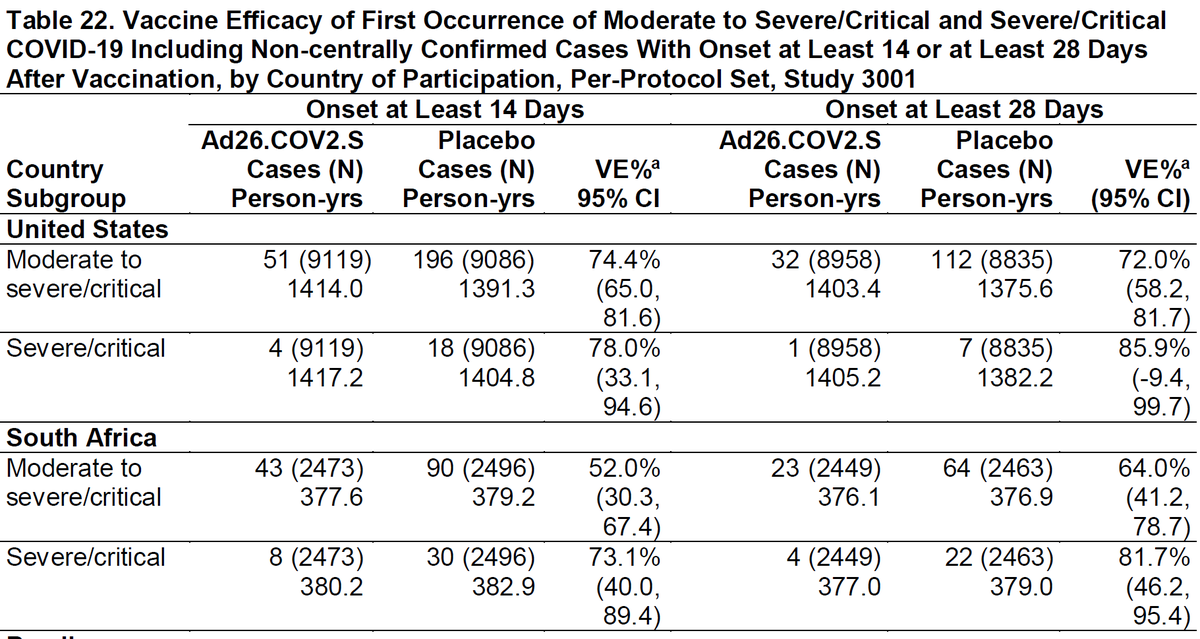
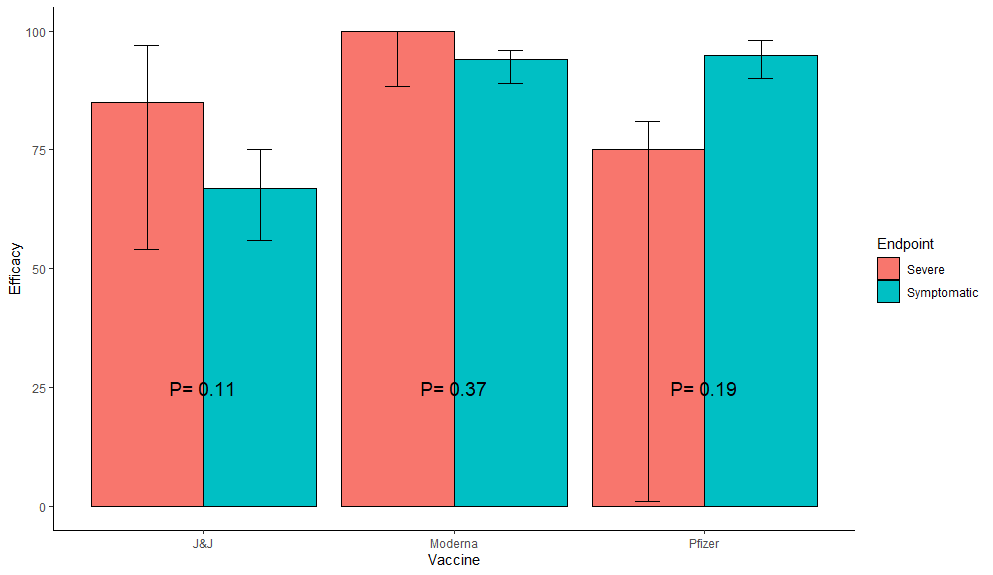
1st: RCTs: No strong evidence for any vaccine having higher efficacy for severe cases (Fisher's exact test P-values); 2 trending higher, 1 trending lower, all CIs large for severe.
Moderna: DOI: 10.1056/NEJMoa2035389
Pfizer: DOI: 10.1056/NEJMoa2034577
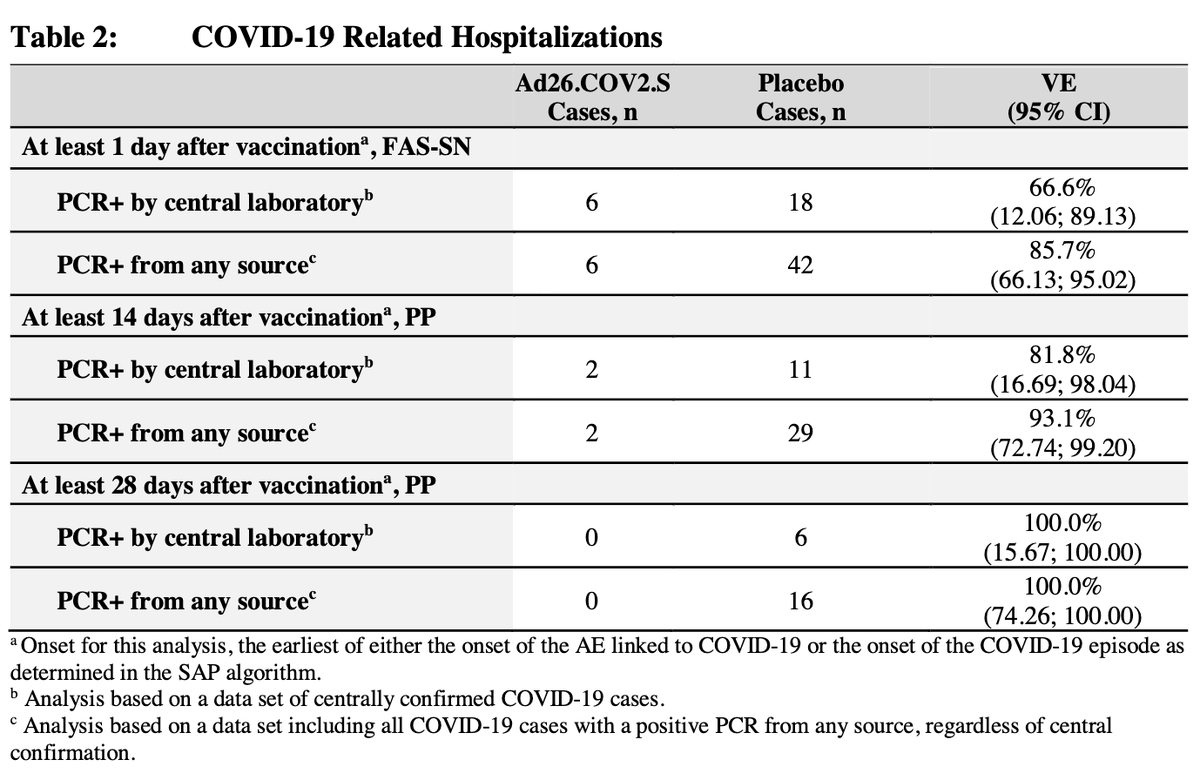
J&J: fda.gov/advisory-commi…
Moderna: DOI: 10.1056/NEJMoa2035389
Pfizer: DOI: 10.1056/NEJMoa2034577
J&J: fda.gov/advisory-commi…
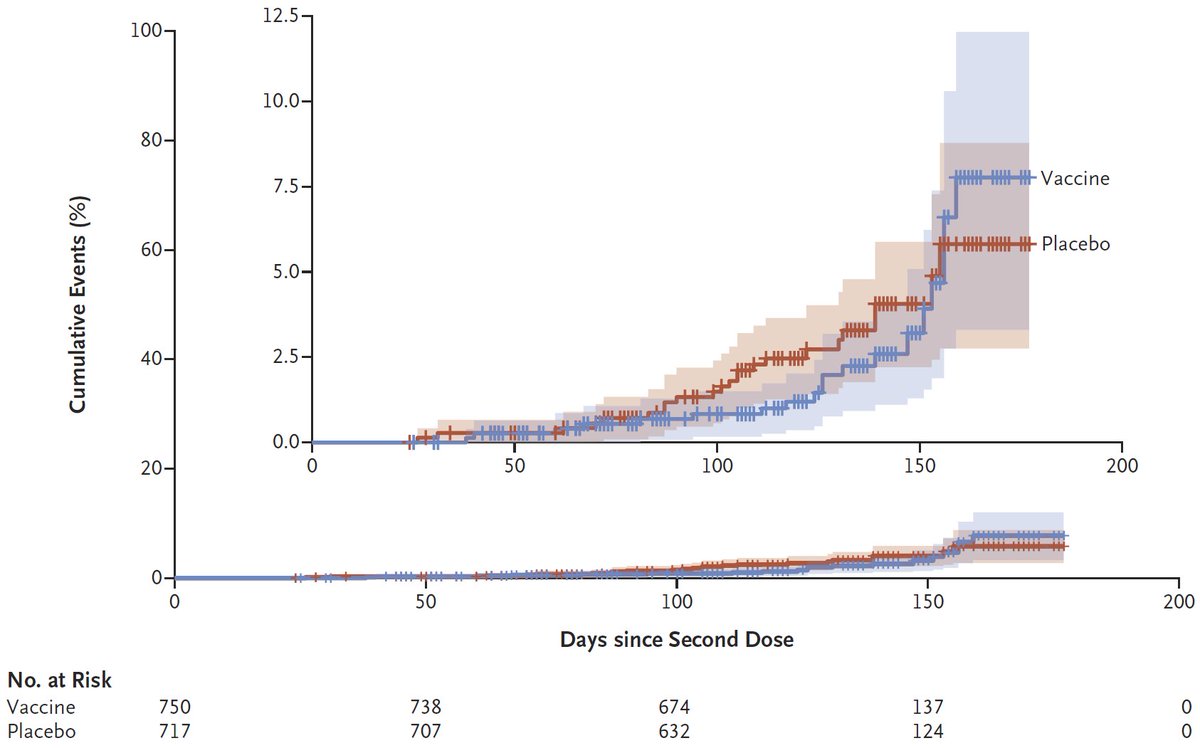
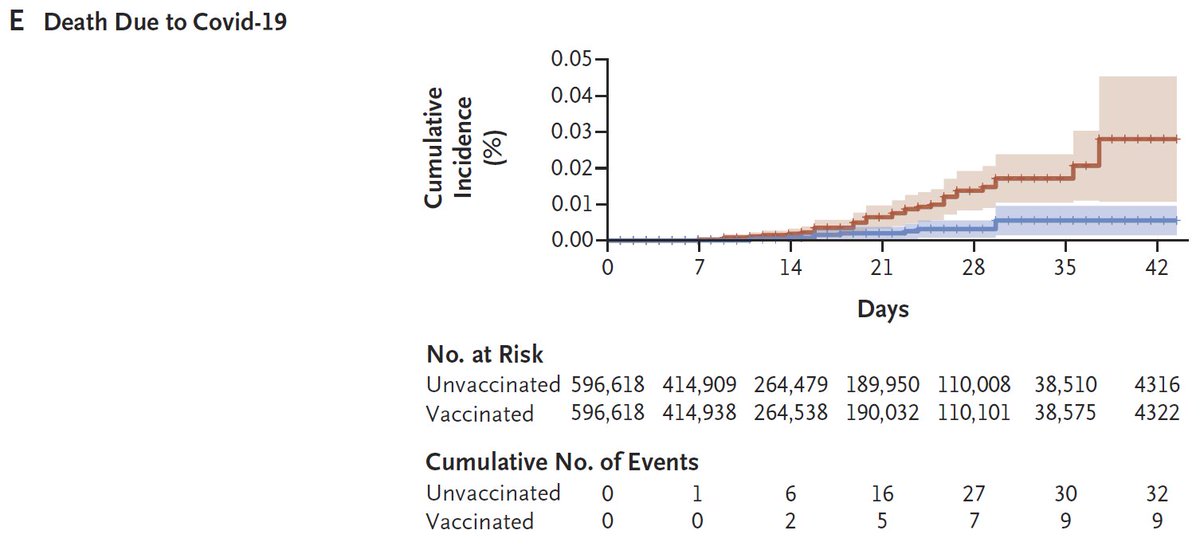
2nd: Effectiveness for Pfizer in Israel (6K events! 369 hosp), w/ 4 disease severities D>Se>H>Sy. After 1st dose, trend? for higher protection for more severe disease, but after 2nd dose trend reversed (no death data post 2nd dose). Again 95% CIs large.
DOI: 10.1056/NEJMoa2101765
DOI: 10.1056/NEJMoa2101765
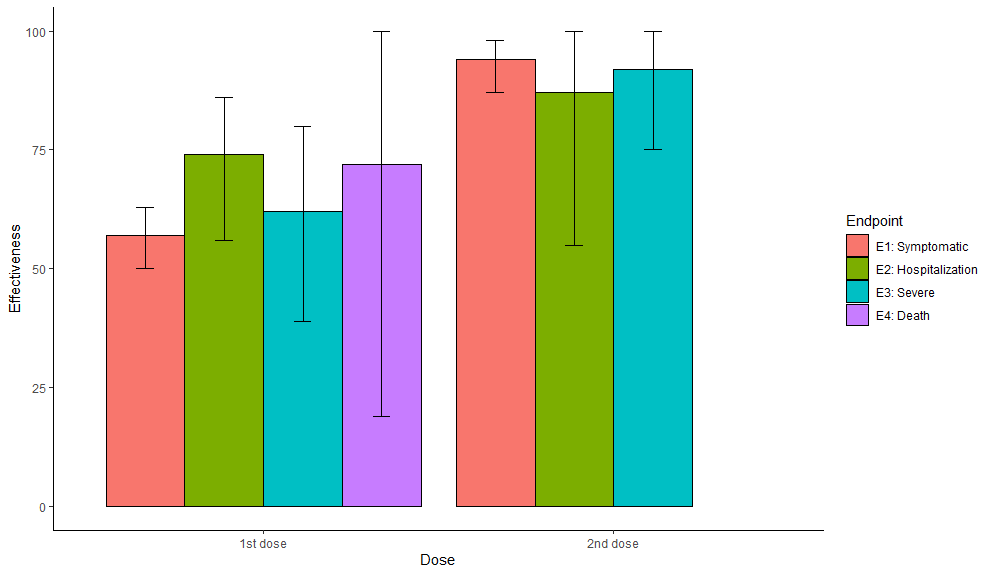
What does it mean? To me it means we should not assume protection against severe disease will always be higher than for "symptomatic disease" (which is mostly composed of mild cases). If protection against mild cases is very low, it MAY be equally low for severe disease. Or not.
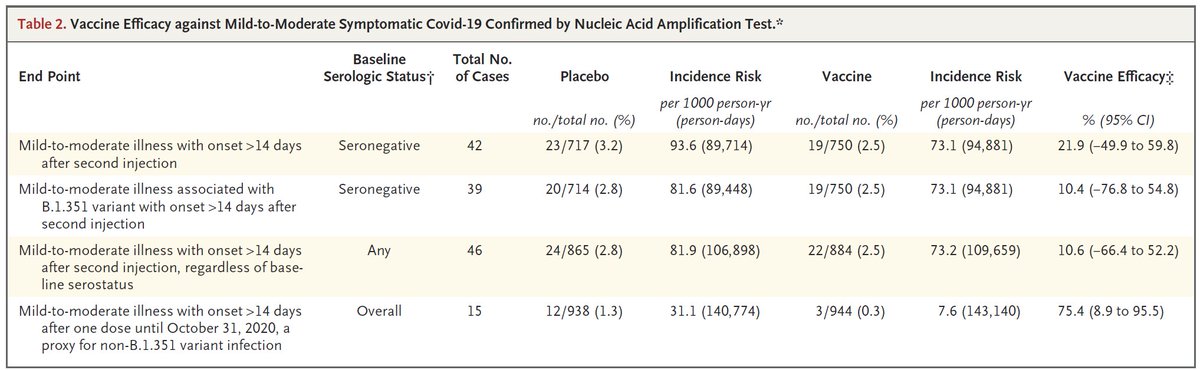
Specifically, AZ had 10.4% (CI: -77%,+55%) efficacy against "mild-moderate" cases of B.1.351. Would efficacy against severe disease be similarly low (e.g. 10%) or much higher (50%+)? If 10% I wouldn't use AZ anywhere B.1.351 was present. If 50%+ I would.
https://twitter.com/DiseaseEcology/status/1371952030424518656
This will be especially important as we study vaccine effectiveness against new variants that have exhibited some immune escape (e.g. B.1.351, P.1, P.2, those w/ E484K mutation). I hope protection against severe disease is higher than for mild, but I'll need data to convince me.
Caveats:
-efficacy against "symptomatic disease" was very high in some cases (~95%), so hard to be higher than that. But in one case (Pfizer RCT), efficacy almost significantly LOWER for severe disease & in Israel, protection very mixed for 4 categories of severity.
-efficacy against "symptomatic disease" was very high in some cases (~95%), so hard to be higher than that. But in one case (Pfizer RCT), efficacy almost significantly LOWER for severe disease & in Israel, protection very mixed for 4 categories of severity.
-Better analysis would have been Poisson regression w/ offset for person-years of exposure, but not all RCTs reported this (that I could find). Exposure time same for different disease severity for each RCT, so not huge issue, but power might be different.
Are there other data I've missed? I purposely left out Astrazeneca data from 1st RCT b/c of mixing of dosing, duration; small number of hospitalizations (10,0) would have produced similar results to that shown above for other RCTs - trend for higher protection but not quite sig.
• • •
Missing some Tweet in this thread? You can try to
force a refresh