
A blockbuster #preprint of #LongCovid from UK; 1077 patients discharged in 2020; at median 5 mos only 29% fully recovered, 20% had new disability. medrxiv.org/content/10.110… @medrxivpreprint @REvans_Breathe @uol3i @HamishMcAuley @LouiseVwain @Survivor_Corps @patientled @dianaberrent 

@medrxivpreprint @REvans_Breathe @uol3i @HamishMcAuley @LouiseVwain @Survivor_Corps @patientled @dianaberrent And another indicator of the impact of #COVID19… at a median of 5 months after hospital discharge, 19% experienced a health-related change in occupation. @medrxivpreprint 

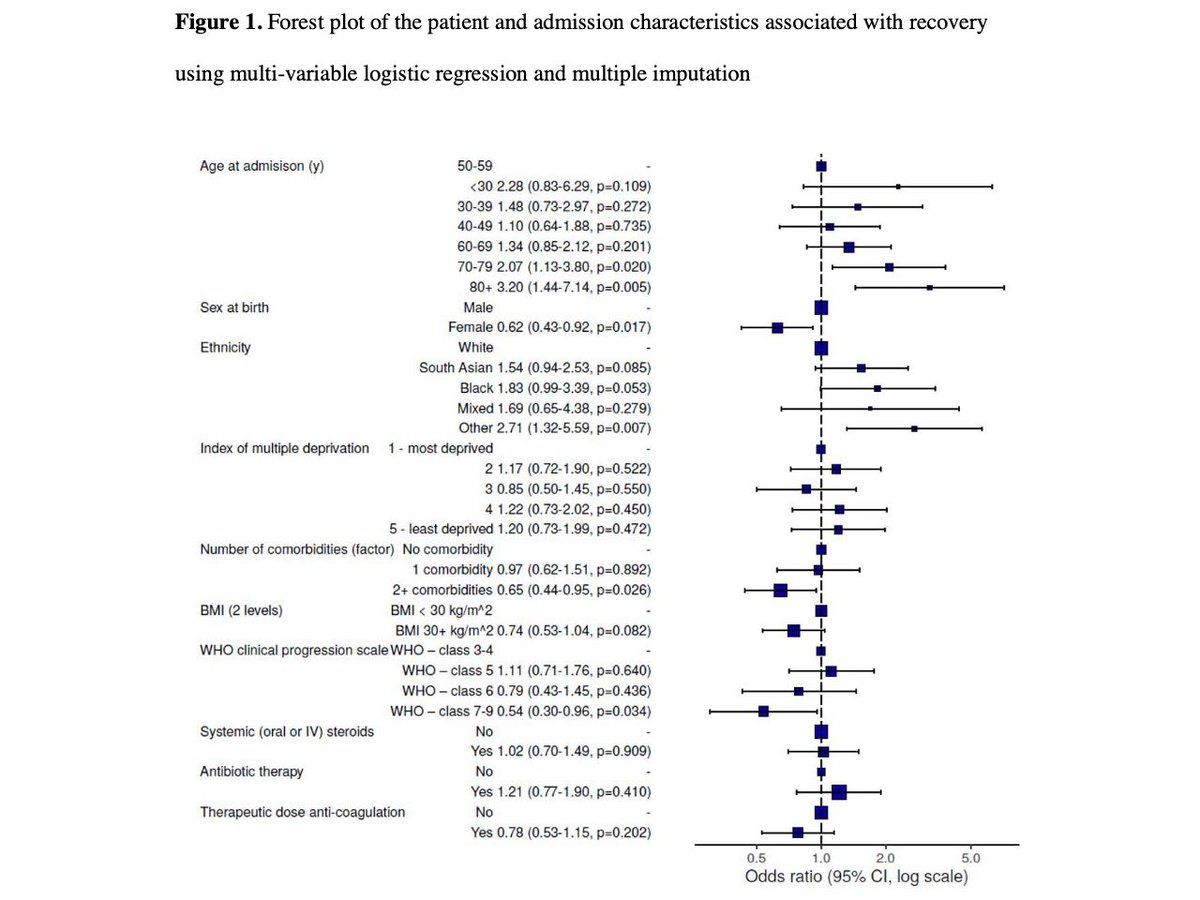
@medrxivpreprint @REvans_Breathe @uol3i @HamishMcAuley @LouiseVwain @Survivor_Corps @patientled @dianaberrent "Factors associated with failure to recover were female, middle-age, white ethnicity, two or more co-morbidities, and more severe acute illness.” 
@medrxivpreprint @REvans_Breathe @uol3i @HamishMcAuley @LouiseVwain @Survivor_Corps @patientled @dianaberrent “The 10 most commonly reported symptoms: ‘aching...muscles (pain)’, ‘fatigue’, ‘physical slowing down’, ‘impaired sleep quality’, ‘joint pain or swelling’, ‘limb weakness’, ‘breathlessness’, ‘pain’, ‘short-term memory loss’ & ‘slowing down in ...thinking’.” @medrxivpreprint
@medrxivpreprint @REvans_Breathe @uol3i @HamishMcAuley @LouiseVwain @Survivor_Corps @patientled @dianaberrent Important that this prospective study had some selection: 'This analysis is restricted to participants who consented to attend two follow-up research visits within one-year post-discharge in addition to routine clinical care.’
@medrxivpreprint @REvans_Breathe @uol3i @HamishMcAuley @LouiseVwain @Survivor_Corps @patientled @dianaberrent "At least a fifth reached the threshold for a new disability with >= 1 domain coded as “a lot of difficulty” or “cannot do it all”… 56.2%, 48.1%, 41.8%, and 38.7% of the cohort reported significant worse symptoms of fatigue, breathlessness, sleep, and pain, respectively."
@medrxivpreprint @REvans_Breathe @uol3i @HamishMcAuley @LouiseVwain @Survivor_Corps @patientled @dianaberrent They identified 4 clusters of #LongCovid 1: v severe mental & physical health impairment (17%); 2 severe mental & physical health impairment (21%);3 mod mental & physical health impairment w/pronounced cognitive impairment; 4: mild mental & physical health impairment (46%). 

• • •
Missing some Tweet in this thread? You can try to
force a refresh












