[THREAD]
After re-listening to parts of Panel Meeting, I think it is safe to assume:
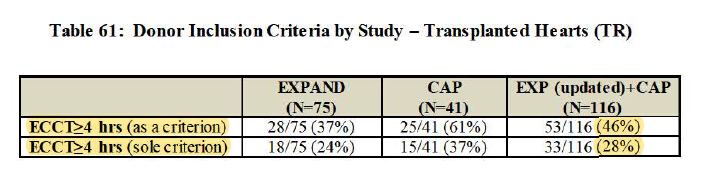
- $TMDX would only be used for extended-criteria hearts only. For ex. >=4hrs ECCT
- Existing SOC volume won't convert over to OCS (unless run back PROCEED Trial to prove OCS better than SOC)
After re-listening to parts of Panel Meeting, I think it is safe to assume:
- $TMDX would only be used for extended-criteria hearts only. For ex. >=4hrs ECCT
- Existing SOC volume won't convert over to OCS (unless run back PROCEED Trial to prove OCS better than SOC)

1/
Updated U.S. TAM table above...
- Total Donors (DBD + DCD) same as previous version (30.4K) per $TMDX 10K
- "Already utilized" organs now reflect actual '20 OPTN data (instead of back-calc from 10K)
- Split in unutilized organs between DBD and DCD extrapolated from 10K
Updated U.S. TAM table above...
- Total Donors (DBD + DCD) same as previous version (30.4K) per $TMDX 10K
- "Already utilized" organs now reflect actual '20 OPTN data (instead of back-calc from 10K)
- Split in unutilized organs between DBD and DCD extrapolated from 10K
2/
- 'Newly-Enabled Organs TAM' excludes 'already utilized' organs (assuming that what can be done today w/ SOC will STILL use SOC... not $TMDX OCS)
- Newly-enabled TAM (all organs) for this reduced by 21% from Full TAM which includes 'Already Utilized' volumes
- 'Newly-Enabled Organs TAM' excludes 'already utilized' organs (assuming that what can be done today w/ SOC will STILL use SOC... not $TMDX OCS)
- Newly-enabled TAM (all organs) for this reduced by 21% from Full TAM which includes 'Already Utilized' volumes
3/
- Math uses 81% Extra Utilization in DBD Heart per EXPAND trial but can actually flex to 84% per EXPAND + CAP... either way still around $2.9Bn (Main star in the show is DCD)
- Again these numbers don't include international (rest of Key Markets) or potential Kidney biz
- Math uses 81% Extra Utilization in DBD Heart per EXPAND trial but can actually flex to 84% per EXPAND + CAP... either way still around $2.9Bn (Main star in the show is DCD)
- Again these numbers don't include international (rest of Key Markets) or potential Kidney biz
4/
- Must apply haircut to TAM b/c a chunk of ppl die @ home (can't get to them till too late), but National Program logistics mapping to partially offset this (would snowball w/ more regions)
- A lot remains to be seen re: how surgeons use OCS if approved (indications etc.)
- Must apply haircut to TAM b/c a chunk of ppl die @ home (can't get to them till too late), but National Program logistics mapping to partially offset this (would snowball w/ more regions)
- A lot remains to be seen re: how surgeons use OCS if approved (indications etc.)
5/ Our view is that demand can be explosive.
Say for hearts, 6.5mm ppl in U.S. have heart failure w/ 5-10% of patients in advanced/end-stage.
That equals 487.5K potential recipients (at midpoint).
Judging from this alone, the supply should be filled if approved.
Say for hearts, 6.5mm ppl in U.S. have heart failure w/ 5-10% of patients in advanced/end-stage.
That equals 487.5K potential recipients (at midpoint).
Judging from this alone, the supply should be filled if approved.

6/ In 2020, 12,588 donors died and donated >=1 organ, while there were only 3,722 Heart Transplants.
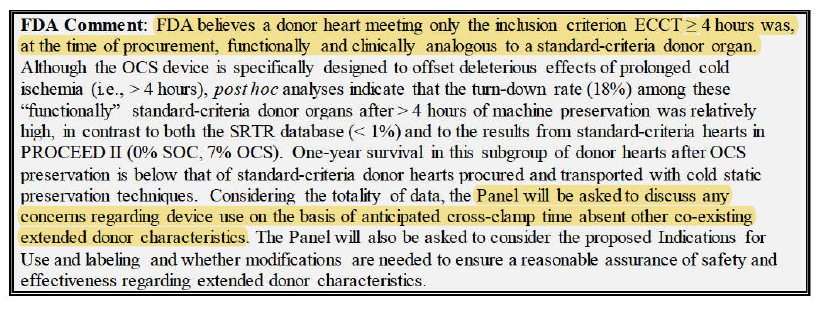
In response to Al Stammers on FDA Panel the other day, CEO Waleed confirmed that w/ just DBD alone (using EXPAND+CAP extra utilization)...
Transplant volumes can double.
In response to Al Stammers on FDA Panel the other day, CEO Waleed confirmed that w/ just DBD alone (using EXPAND+CAP extra utilization)...
Transplant volumes can double.

7/ Our math gets to 4,454 extra hearts or 2.2x today's volume.
If can achieve, that's $200mm in sales for that alone ($45K/Tx).
DCD will then open pool to potential 16,943 hearts. If achieve, that's another $762mm.
(All of this while disregarding already utilized hearts)
If can achieve, that's $200mm in sales for that alone ($45K/Tx).
DCD will then open pool to potential 16,943 hearts. If achieve, that's another $762mm.
(All of this while disregarding already utilized hearts)
8/ DCD Heart will have readout of top line data
- May / may not have Panel meeting (has breakthrough designation, so potential streamline process)
- Expect to file PMA in Q3'21
- Approval probably 9-12mo post-file (so '22)
By that time, National Program likely very strong.
- May / may not have Panel meeting (has breakthrough designation, so potential streamline process)
- Expect to file PMA in Q3'21
- Approval probably 9-12mo post-file (so '22)
By that time, National Program likely very strong.
9/ Moreover, all the math drive off 30.4K assumption of donors per $TMDX 10K.
IMO 30.4K sounds low given 2.8mm deaths a year in U.S. + 165mm signed up to be organ donors.
Filtering down w/ CDC by age, place of death, etc... 30.4K still looks small.
IMO 30.4K sounds low given 2.8mm deaths a year in U.S. + 165mm signed up to be organ donors.
Filtering down w/ CDC by age, place of death, etc... 30.4K still looks small.
https://twitter.com/genghis_pon/status/1376536397503365126?s=21
10/ DBD + DCD newly enabled hearts give sales opp of $962mm total.
If you apply haircut of 50% assuming death @ home, families refusing to sign off, logistics issues...
It's still $480mm for just U.S. hearts.
Haircut gets smaller as National Program gets efficient.
If you apply haircut of 50% assuming death @ home, families refusing to sign off, logistics issues...
It's still $480mm for just U.S. hearts.
Haircut gets smaller as National Program gets efficient.
11/ National Program can help $TMDX strong-arm competitors so I have fewer concerns about other players gaining lots of U.S. share.
At $33.50/share, I have EV of $884.7mm using FDSO.
10.2x EV/'22 Sales
5.9x EV/'23 Sales
Execution matters & have to keep retesting. That said...
At $33.50/share, I have EV of $884.7mm using FDSO.
10.2x EV/'22 Sales
5.9x EV/'23 Sales
Execution matters & have to keep retesting. That said...
12/ Don't think $TMDX is expensive but my time-frame is long. My views only.
I think 22K DCD deaths of which 16.9K hearts can potentially be used by OCS is a huge number / sales opp that will be a landslide when approved.
DCD donor hearts possible only b/c of $TMDX.
I think 22K DCD deaths of which 16.9K hearts can potentially be used by OCS is a huge number / sales opp that will be a landslide when approved.
DCD donor hearts possible only b/c of $TMDX.
13/ That alone gives $762mm total sales opp.
In addition, surgeons would embrace DCD hearts.
It won't be on the fence.
There's no competition.
I reckon the ramp up will be much FASTER than we expect for DCD b/c there's no other choice and can provide immediate benefit.
In addition, surgeons would embrace DCD hearts.
It won't be on the fence.
There's no competition.
I reckon the ramp up will be much FASTER than we expect for DCD b/c there's no other choice and can provide immediate benefit.
14/ OPTN has multiple data pulls which spit out similar numbers but are actually different.
This table below now exactly ties the # of utilized hearts to $TMDX's sponsor presentation.
DBD Opp $201mm
DCD Opp $764mm
Newly-Enabled Organ U.S. TAM $3.0Bn vs. $2.9Bn in 1st slide.

This table below now exactly ties the # of utilized hearts to $TMDX's sponsor presentation.
DBD Opp $201mm
DCD Opp $764mm
Newly-Enabled Organ U.S. TAM $3.0Bn vs. $2.9Bn in 1st slide.
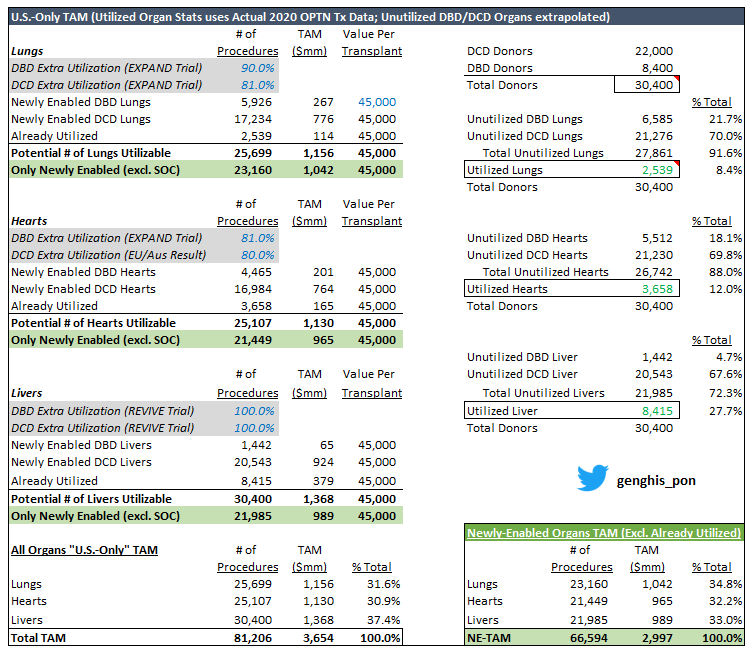

Please look at last tweet in thread for a revised table with 3,658 hearts and the corresponding outputs.
OPTN has a lot of data and I linked a different row to populate first table up top. Would recommend looking at last table in thread instead for 100% clarity.
OPTN has a lot of data and I linked a different row to populate first table up top. Would recommend looking at last table in thread instead for 100% clarity.
This 50% haircut can presumably include scenarios where Tx surgeon choose to wait longer to use SOC donor heart nearby instead of OCS extended-criteria heart.
As noted on Panel, there will be cases where Tx surgeons prefer to harvest hearts locally w/ SOC to get 95% survival.
As noted on Panel, there will be cases where Tx surgeons prefer to harvest hearts locally w/ SOC to get 95% survival.
• • •
Missing some Tweet in this thread? You can try to
force a refresh