
Sputnik V has 91.6% efficacy against COVID-19 & 100% against severe covid. 5 countries producing domestically incl. India & China. Aka GAM-COVID-Vac, it’s made of recombinant (S protein expressing gene of SARS-CoV2) adenovirus of 2 different serotypes (26 & 5)
Approved in India✅
Approved in India✅
PS it’s not Sputnik 5, it’s Sputnik V (the alphabet) probably a short to suggest it’s a viral vector vaccine. Sputnik 1 was also the first human-made satellite to orbit the earth.
Sputnik V is a double dose, 21 days apart vaccine. Both doses contain different serotypes of adenovirus. Single dose is not so effective (~85%). 2 way storage: 1. As a ready made water solution stored at -18•C OR 2. the freeze dried powder stored at 2-8•C & later mixed in water
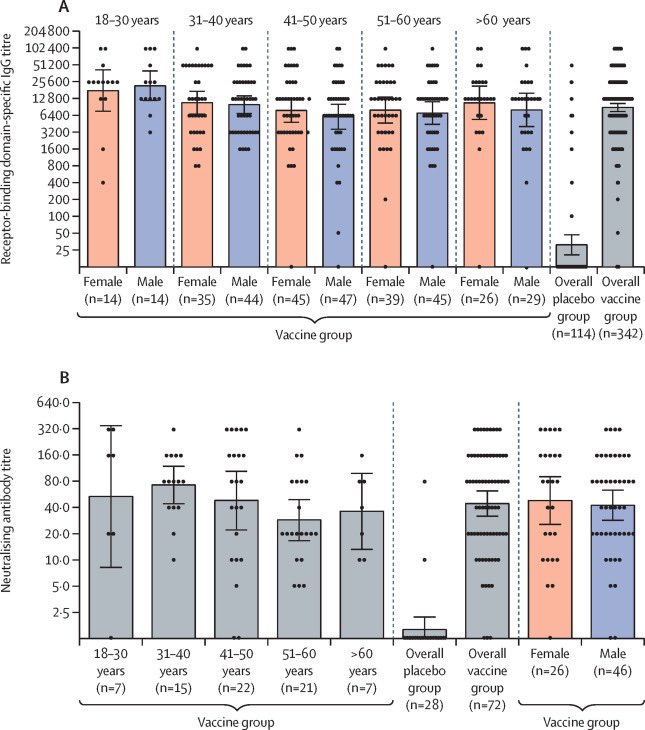
Sputnik reported no serious adverse effects related to vaccination, except grade 1 events (flu, headache,etc). Serious effects reported in both placebo & vaccine groups. Total in phase ||| study ~20K. Data shows gender & ethnic distribution @TheLancet
thelancet.com/journals/lance…
thelancet.com/journals/lance…
• • •
Missing some Tweet in this thread? You can try to
force a refresh




