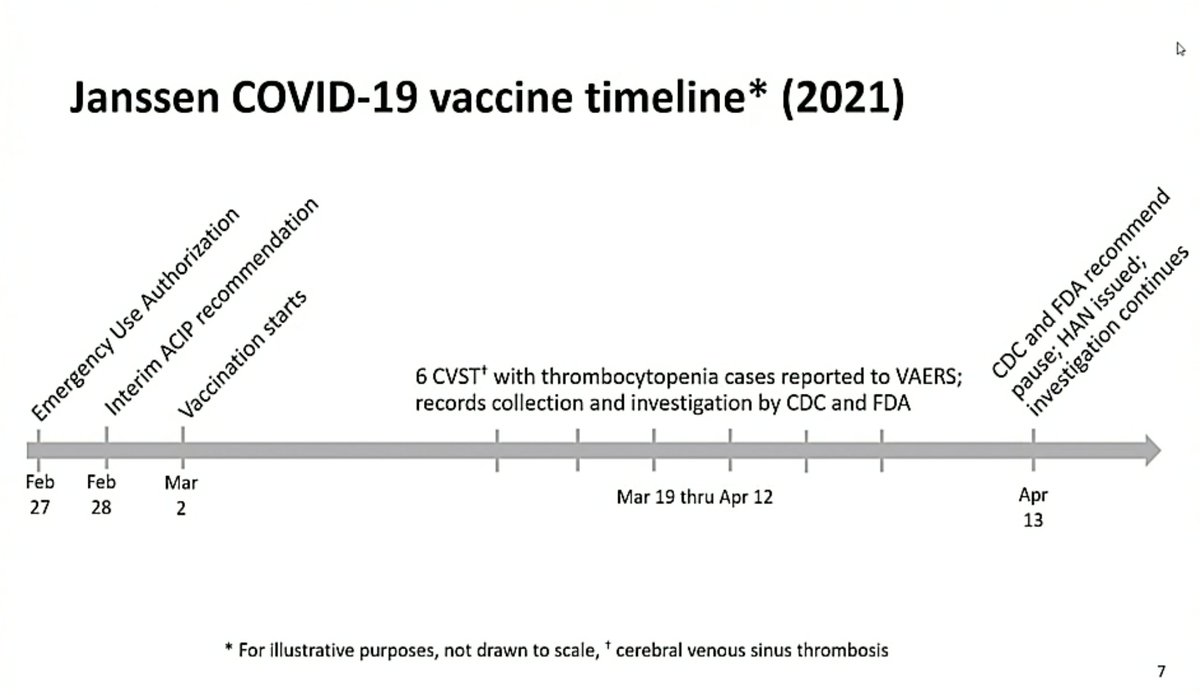
Most fascinating bit of ACIP meeting so far is a detail on the 25-year old male in J&J trial, who developed CVST with hemorrhage after 8 days.
J&J representative says it was retrospectively determined that he was negative for anti-PF4 antibodies before vaccination, positive after
J&J representative says it was retrospectively determined that he was negative for anti-PF4 antibodies before vaccination, positive after
Case reports are fascinating.
Here are some details on the case from previous tweet.
(Short sentence on anti-PF4 antibodies is a bit misleading here: he was negative at baseline, positive post-vaccination according to the presentation)
Here are some details on the case from previous tweet.
(Short sentence on anti-PF4 antibodies is a bit misleading here: he was negative at baseline, positive post-vaccination according to the presentation)

And here is a comparison of the three vaccines in use in the US for CVST.
As you can see there are no cases of CVST with thrombocytopenia reported for the two mRNA vaccines.
As you can see there are no cases of CVST with thrombocytopenia reported for the two mRNA vaccines.

Some characteristics of the six reported cases of CVST with thrombocytopenia after J&J vaccine.
Only one patient was using estrogen/progesterone, none had reported coagulation disorders.
Only one patient was using estrogen/progesterone, none had reported coagulation disorders.

“The important thing to note here is these initial features are largely nonspecific symptoms…
I do think it's important in the setting we are right now, that healthcare providers maintain a high index of suspicion for possible CVST”
I do think it's important in the setting we are right now, that healthcare providers maintain a high index of suspicion for possible CVST”

“We think it's important to look for substantial blood clots in individuals with thrombocytopenia regardless of whether they're in the CNS or not.” 

Here we go. Was hoping to hear about anti-PF antibodies:
At least 5 patients did have these antibodies.
At least 5 patients did have these antibodies.

And here is a comparison of cases expected and observed in women aged 20-50 (for different assumed background rates).
This, basically, is the safety signal.
This, basically, is the safety signal.

(Notes afterwards that these expected numbers are based on CVST in general, not CVST with thrombocytopenia. So expected numbers here are very possibly an overestimate, making safety signal even stronger.)
And I’ll just post the summary slides here as well:
(Emphasizes again not to use heparin in patients with thromboses after J&J vaccination, unless HIT test is negative.
Also emphasizes how important the reporting of adverse events via VAERS is.)


(Emphasizes again not to use heparin in patients with thromboses after J&J vaccination, unless HIT test is negative.
Also emphasizes how important the reporting of adverse events via VAERS is.)



Here we go. Was hoping to hear about anti-PF antibodies:
At least 5 patients did have these antibodies.
At least 5 patients did have these antibodies.

And here is a comparison of cases expected and observed in women aged 20-50 (for different assumed background rates).
This, basically, is the safety signal.
This, basically, is the safety signal.

(Notes afterwards that these expected numbers are based on CVST in general, not CVST with thrombocytopenia. So expected numbers here are very possibly an overestimate, making safety signal even stronger.)
And I’ll just post the summary slides here as well:
(Emphasizes again not to use heparin in patients with thromboses after J&J vaccination, unless HIT test is negative.
Also emphasizes how important the reporting of adverse events via VAERS is.)


(Emphasizes again not to use heparin in patients with thromboses after J&J vaccination, unless HIT test is negative.
Also emphasizes how important the reporting of adverse events via VAERS is.)



Side note:
There was a lot of criticism yesterday of CDC/FDA for their decision. Won’t get into that here.
But after following essentially the same debate in Europe on AZ for a month now (EMA/PEI mostly), this meeting is next level in terms of transparency. We need more of this.
There was a lot of criticism yesterday of CDC/FDA for their decision. Won’t get into that here.
But after following essentially the same debate in Europe on AZ for a month now (EMA/PEI mostly), this meeting is next level in terms of transparency. We need more of this.
Some very important background data now presented by Sara Oliver as we come to benefit/risk debate.
Most importantly:
- About 1,5 million doses administered to women 18-50 years of age
- About 1/2 of 7 million doses administered before end of March (so CVST likely seen already)


Most importantly:
- About 1,5 million doses administered to women 18-50 years of age
- About 1/2 of 7 million doses administered before end of March (so CVST likely seen already)



And two good slides outlining what IS known and what is NOT known.
We often talk about decisions having to be made amid uncertainty in situations like this. Absolutely fascinating to see this play out in public in real life.

We often talk about decisions having to be made amid uncertainty in situations like this. Absolutely fascinating to see this play out in public in real life.


Basic policy options now are:
- stop using J&J
- use it only in some populations (only men, only people aged 50+)
- restart use in all adults
(@DrPaulOffit outlined this in our story yesterday too: sciencemag.org/news/2021/04/c…)
- stop using J&J
- use it only in some populations (only men, only people aged 50+)
- restart use in all adults
(@DrPaulOffit outlined this in our story yesterday too: sciencemag.org/news/2021/04/c…)

@DrPaulOffit Interesting point raised in the debate just now is that most US hospitals probably do not have the ability to test for HIT in-house, so would have to send out for this (and demand for these tests may well rise).
@DrPaulOffit Public comments now. First person called was antivaxxer DelBigtree (read @deerbrian’s great book on Wakefield for more) but didn’t answer, so moved on to next.
@DrPaulOffit @deerbrian (One more side note:
I get annoyed at US centric view of issues sometimes and was impressed that in outlining possible impact of restricting/stopping J&J, global repercussions were included)
I get annoyed at US centric view of issues sometimes and was impressed that in outlining possible impact of restricting/stopping J&J, global repercussions were included)

@DrPaulOffit @deerbrian Antivaxxer DelBigtree now, unsurprisingly:
“Please do the CDC a favor, do this nation a favor, and lead by erring on the side of caution for the citizens, and not the pharmaceutical industry, and pull Johnson and Johnson immediately."
“Please do the CDC a favor, do this nation a favor, and lead by erring on the side of caution for the citizens, and not the pharmaceutical industry, and pull Johnson and Johnson immediately."
@DrPaulOffit @deerbrian (While there is a lot of worry that anti-vaxxers will use this safety signal for their aims, this whole process - for anyone who actually follows it - should reassure people that these safety signals are picked up and are taken seriously. Important to report the process here!)
@DrPaulOffit @deerbrian So questions now for voting members:
Is there enough information to make interim age or risk-factor based recommendations for this vaccine?
What recommendation does ACIP feel is appropriate today given current available information for use of the Janssen vaccine?
Is there enough information to make interim age or risk-factor based recommendations for this vaccine?
What recommendation does ACIP feel is appropriate today given current available information for use of the Janssen vaccine?

@DrPaulOffit @deerbrian Sounds like a lot of support for the option of continuing the J&J immunization pause while more data is gathered.
@DrPaulOffit @deerbrian Important point raised by @KottonNelson:
At least in Massachusetts this “one-and-done” shot was to be used "for our vulnerable inpatient populations, often with many comorbidities and at high risk for disease but who haven't been able to get vaccinated otherwise"
At least in Massachusetts this “one-and-done” shot was to be used "for our vulnerable inpatient populations, often with many comorbidities and at high risk for disease but who haven't been able to get vaccinated otherwise"
@DrPaulOffit @deerbrian @KottonNelson Echoed by @nirav_mainecdc:
“not making a decision is tantamount to making a decision, and the extension of the pause will invariably result in the fact that the most vulnerable individuals in United States who were prime candidates of J&J vaccine will remain vulnerable"
“not making a decision is tantamount to making a decision, and the extension of the pause will invariably result in the fact that the most vulnerable individuals in United States who were prime candidates of J&J vaccine will remain vulnerable"
@DrPaulOffit @deerbrian @KottonNelson @nirav_mainecdc Interesting point by Helen Keipp Talbot:
Those who are most vulnerable may also be at special risk from severe clotting and may not get care on time, so “a double-edged sword”.
Those who are most vulnerable may also be at special risk from severe clotting and may not get care on time, so “a double-edged sword”.
@DrPaulOffit @deerbrian @KottonNelson @nirav_mainecdc It’s over!
Decision is not to vote on any changes to the recommendations today and to continue the pause while more data is gathered and then to meet again in a week or ten days.
(Technically, I think, they are simply not making any recommendation to CDC director.)
Decision is not to vote on any changes to the recommendations today and to continue the pause while more data is gathered and then to meet again in a week or ten days.
(Technically, I think, they are simply not making any recommendation to CDC director.)
@DrPaulOffit @deerbrian @KottonNelson @nirav_mainecdc Not the most exciting RESULT and this will lead to a lot of debate, but I have to say that this was an amazing display of the PROCESS of vaccine safety (and yes, we journalists are terrible at writing about/explaining process in an engaging way, though it is so important.)
@DrPaulOffit @deerbrian @KottonNelson @nirav_mainecdc Sorry everyone for a thread, that was a bit denser than usual.
Hard to break down information while listening and live-tweeting.
I do think the data presented here give us a lot of context we were missing.
Hard to break down information while listening and live-tweeting.
I do think the data presented here give us a lot of context we were missing.
@DrPaulOffit @deerbrian @KottonNelson @nirav_mainecdc About risk:
For now a lot depends on the denominator you choose.
You can say 6 out of more than 6 million vaccines, so 1 in a million
.
Or if you use female vaccinees aged 18-50 and vaccinated more than 2 weeks ago, it’s more like 6 out of 700,000, so closer to 1 in 100,000
For now a lot depends on the denominator you choose.
You can say 6 out of more than 6 million vaccines, so 1 in a million
.
Or if you use female vaccinees aged 18-50 and vaccinated more than 2 weeks ago, it’s more like 6 out of 700,000, so closer to 1 in 100,000
@DrPaulOffit @deerbrian @KottonNelson @nirav_mainecdc And remember:
-some people vaccinated in last two weeks may only present with symptoms in next days
and
- there may be “stimulated reporting”, so cases found because of the attention drawn to this
So reasonable to expect numbers to go up and those cases will add data to evaluate
-some people vaccinated in last two weeks may only present with symptoms in next days
and
- there may be “stimulated reporting”, so cases found because of the attention drawn to this
So reasonable to expect numbers to go up and those cases will add data to evaluate
@DrPaulOffit @deerbrian @KottonNelson @nirav_mainecdc I’m off to bed and will likely dream of disembodied voices discussing risk-benefit of vaccines.
Meet you back here in a week or ten days, I guess...
Meet you back here in a week or ten days, I guess...
• • •
Missing some Tweet in this thread? You can try to
force a refresh