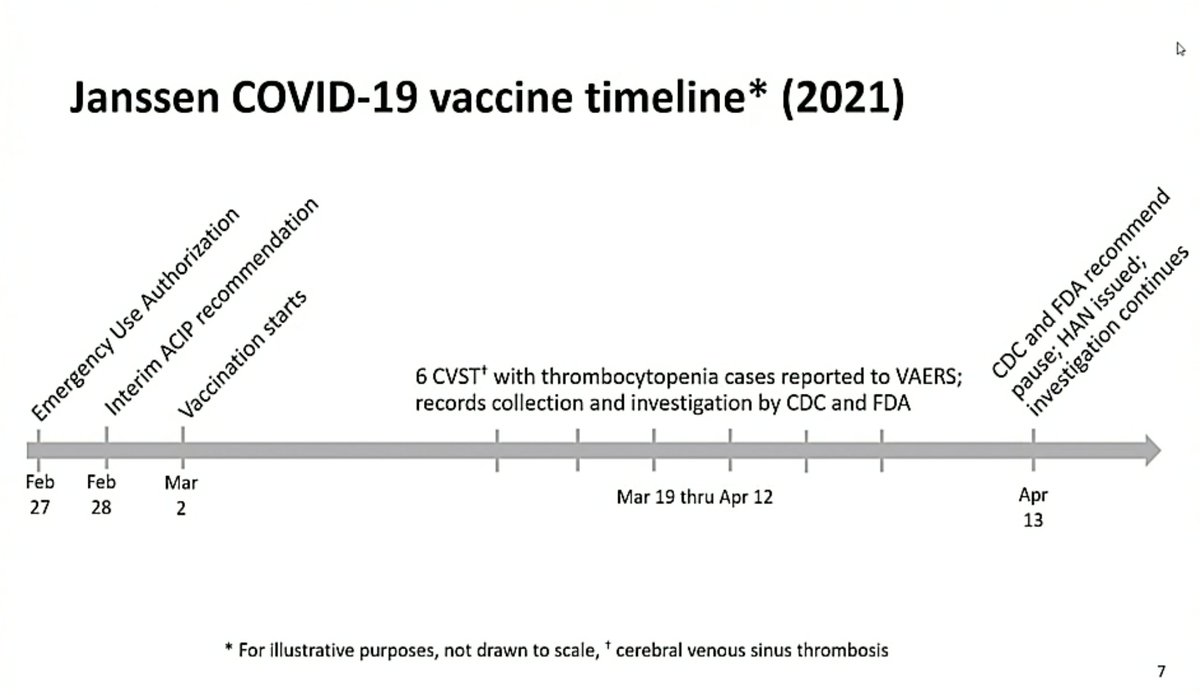
There is an interesting new preprint out that will probably generate a lot of coverage at least in the UK. Essentially it argues that the risk of CVST is much higher from #covid19 than from vaccines.
Quick thread on this:
osf.io/a9jdq/
Quick thread on this:
osf.io/a9jdq/
Here is an image from the paper that is likely to feature heavily in debates around this.
As you can see the risk of CVST here seems to be 8-10 times higher in people with CVST than in people who received mRNA vaccines or AstraZeneca.
BUT: A lot of caveats here.
As you can see the risk of CVST here seems to be 8-10 times higher in people with CVST than in people who received mRNA vaccines or AstraZeneca.
BUT: A lot of caveats here.

First of all:
The paper really only makes a like-with-like comparison with mRNA vaccines (as authors pointed out in presser this morning too: “I think our data say actually nothing about the AZ vaccine.”).
That’s why the data on AstraZeneca is greyed out in that graph.
The paper really only makes a like-with-like comparison with mRNA vaccines (as authors pointed out in presser this morning too: “I think our data say actually nothing about the AZ vaccine.”).
That’s why the data on AstraZeneca is greyed out in that graph.
When you look at mRNA the numbers are very surprising:
The researchers essentially found 2 cases of CVST in 500,000 people that received mRNA vaccine.
Numbers from ACIP yesterday are:
No CVST after 98 million doses of Biontech.
3 cases after 85 million doses of Moderna.
The researchers essentially found 2 cases of CVST in 500,000 people that received mRNA vaccine.
Numbers from ACIP yesterday are:
No CVST after 98 million doses of Biontech.
3 cases after 85 million doses of Moderna.
Hard to reconcile an estimate of 4 in a million, with the reported frequency of 3 in 180 million doses.
I have no good explanation for this. Asked one of the scientists and he wrote: "it may just be chance, or diagnoses coded in error, or using different diagnostic criteria"
I have no good explanation for this. Asked one of the scientists and he wrote: "it may just be chance, or diagnoses coded in error, or using different diagnostic criteria"
We know that #covid19 can lead to blood clotting, so finding cases of CVST in #covid19 patients is not surprising.
The very high number here is surprising and if that is borne out it is interesting and important.
The very high number here is surprising and if that is borne out it is interesting and important.
The main question here, of course, is should this change our risk-benefit-analysis and for now I don’t see that it does.
Reason no. 1: This tells us little about AstraZeneca which according to a lot of data has a much higher risk of CVST than other vaccines.
Reason no. 1: This tells us little about AstraZeneca which according to a lot of data has a much higher risk of CVST than other vaccines.
Reason no.2:
These serious outcomes of #covid19 should be priced into risk-benefit analyses like those visualised by @d_spiegel already since they simply look at overall risk of severe #covid19 in different age groups. (Spiegelhalter confirmed to me that they are).


These serious outcomes of #covid19 should be priced into risk-benefit analyses like those visualised by @d_spiegel already since they simply look at overall risk of severe #covid19 in different age groups. (Spiegelhalter confirmed to me that they are).



@d_spiegel I asked one of the researchers about this.
His reply:
His reply:
@d_spiegel "Whether it alters the risk-benefit picture is a moot point – at least here in the UK, the preoccupation is particularly with the CVT risk not with risks more generally, and so we hope that by drawing attention to this specific outcome after COVID we can help inform the debate"
So I worry that the way this research will be portrayed is going to be very different from it tells us (and what the researchers tell us about it).
But let’s wait and see.
But let’s wait and see.
• • •
Missing some Tweet in this thread? You can try to
force a refresh