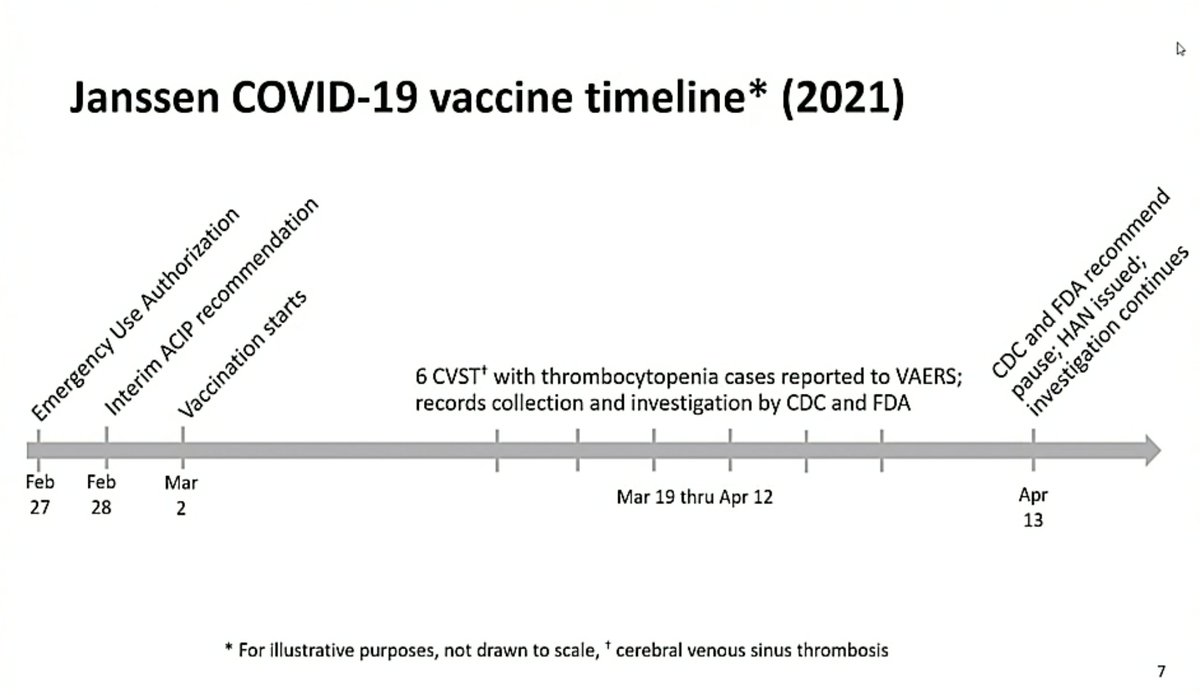
“We have now heard of 8 cases of these very rare side effects as part of the rollout in the US, where the vaccine has been given to over 7 million vaccinees”, says EMA head Emer Cooke at press briefing on the safety review of J&J's #covid19 vaccine.
“This is a very rare effect but it also makes it very important for doctors and patients to be aware of the signs so that they can spot any concerns and seek specialist help as soon as possible”, says Cooke. "Early intervention by specialists can change the outcome."
"The scientific assessment that PRAC has concluded on today will allow vaccination programs in member states to take decisions on how to roll out this vaccine based on their national situation” (infections, hospitalizations, ICU admissions, vaccine availability), says Cooke.
PRAC concluded there is a "possible link" between clotting disorders and the J&J vaccine, says Sabine Straus. “The product information will be updated to reflect this information and will include a warning, and an update of the side effects"
"The careful review of the cases, and other available evidence, have led the committee to the conclusion that these blood clotting disorders are very rare side effects of the vaccine”, says Straus.
"At this moment, it's not possible to identify clear risk factors for the occurrence of these very rare events, such as gender or age”, says Straus.
Sraus lists symptoms to look out for within 3 weeks after vaccination:
shortness of breath
chest pain
swelling in the leg
persistent abdominal pain
neurological symptoms, including severe or persistent headaches or blurred vision
skin bruising beyond the site of injection
shortness of breath
chest pain
swelling in the leg
persistent abdominal pain
neurological symptoms, including severe or persistent headaches or blurred vision
skin bruising beyond the site of injection
"People experiencing any of these symptoms after vaccination should seek medical assistance”, says Straus. “If treated early, healthcare professionals can help those affected in their recovery and avoid further complications."
Q whether this is the same mechanism as with AZ.
“There are quite some similarities between the two vaccines, but there are also differences”, says Straus. Mentions chimp vs. human adenovirus and differences in spike.
“There are quite some similarities between the two vaccines, but there are also differences”, says Straus. Mentions chimp vs. human adenovirus and differences in spike.
“At the moment, we see that the cases have a lot of similarities”, says Straus. "We think that the hypothesis to explain the end of the cascade is probably similar, but it's too early to draw any further conclusions."
Arlett gives a breakdown of cases of blood clots with thrombocytopenia by vaccine:
J&J: 8 cases (all US)
AZ: 287 cases (142 from European Economic Area)
Pfizer: 25 cases
Moderna: 5 case
J&J: 8 cases (all US)
AZ: 287 cases (142 from European Economic Area)
Pfizer: 25 cases
Moderna: 5 case
Straus notes that J&J was paused in US a week ago and that cases can still occur for three weeks after immunization. "So we still have to wait and see if there are more cases coming in, so it's too early to say anything about the real occurrence of the cases."
Sounds like the cases after mRNA vaccines are only from Europe (at least Straus confirms that the US has not seen any cases). Also says that given number of doses administered it is not clear that this is a real safety signal (i.e. it could be coincidence)
So Arlett confirms that the cases after mRNA vaccines he listed were thromboses WITH thrombocytopenia (data lock was 13 April).
He says numbers are from Eudra database and companies are obligated to report all worldwide cases.
He says numbers are from Eudra database and companies are obligated to report all worldwide cases.
I’m sorry to say, but after listening to ACIP meeting last week, this presser by @EMA_News just feels wholly inadequate to me. (And I can see that you can make an argument for experts being in a room and having an open discussion without people listening in, but still…)
@EMA_News Even if you do a presser, I would expect more info up front (maybe even a graphic 😱).
This wholly relies on people asking the right questions. The way this is done does not help to have clear, informed media reports IMHO.
This wholly relies on people asking the right questions. The way this is done does not help to have clear, informed media reports IMHO.
I find it pretty shocking for instance to give numbers for these rare side effects without giving the number of people vaccinated at the same time...
Will put this here too, since this is the data I wanted to hear in the presser:
https://twitter.com/kakape/status/1384776607466938368
• • •
Missing some Tweet in this thread? You can try to
force a refresh