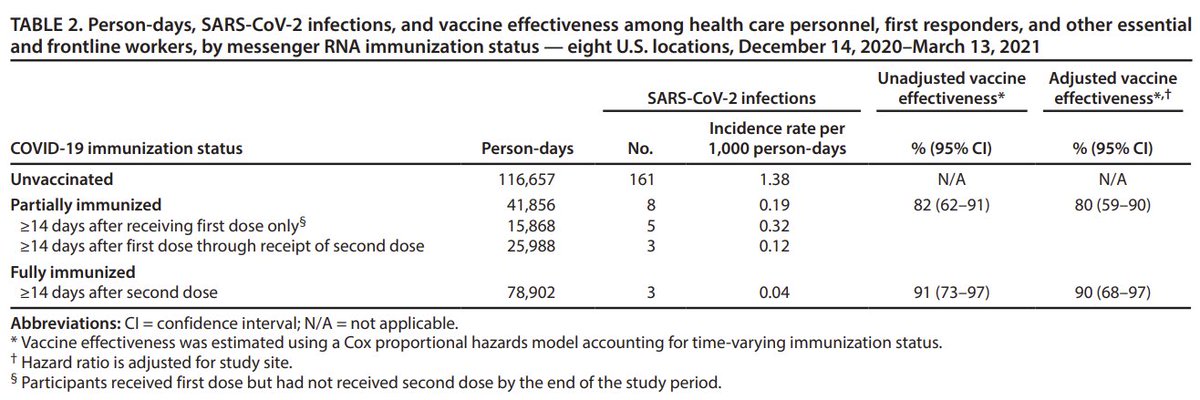
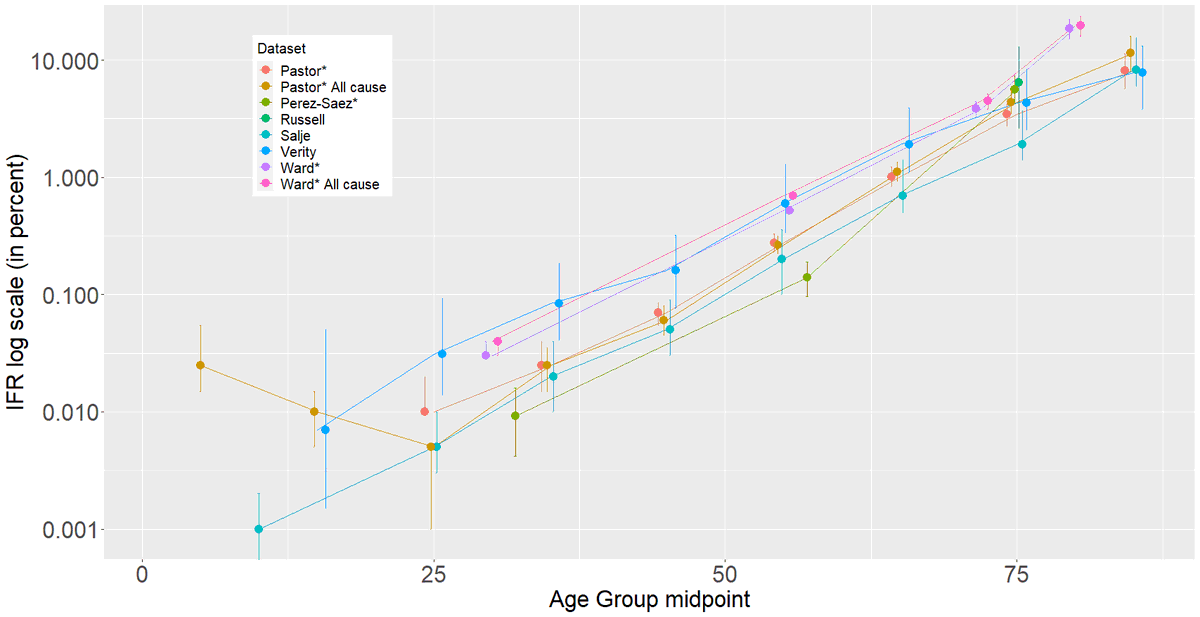
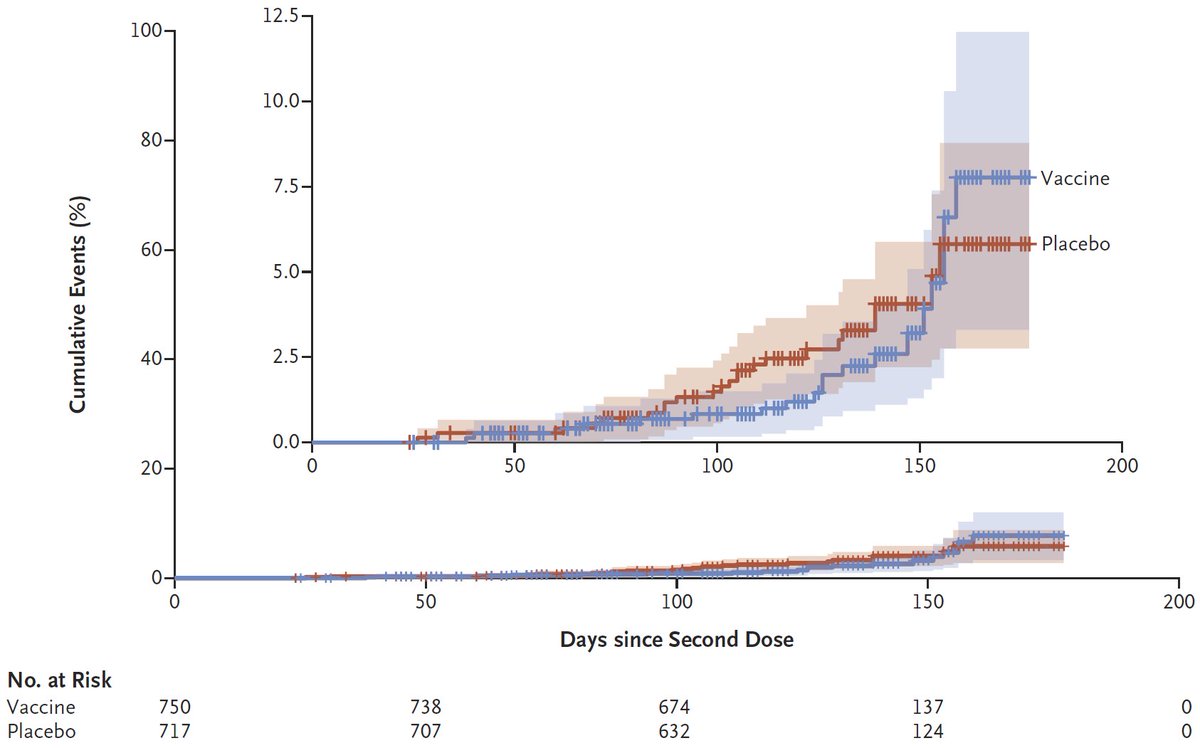
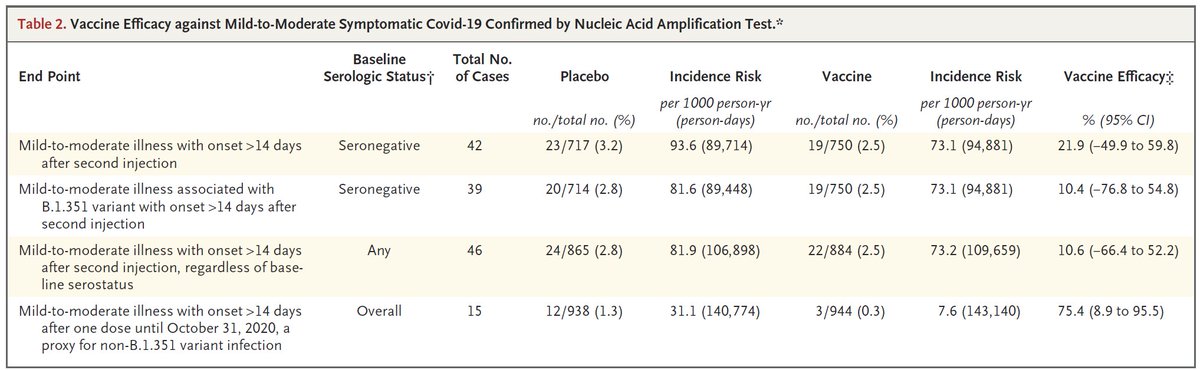
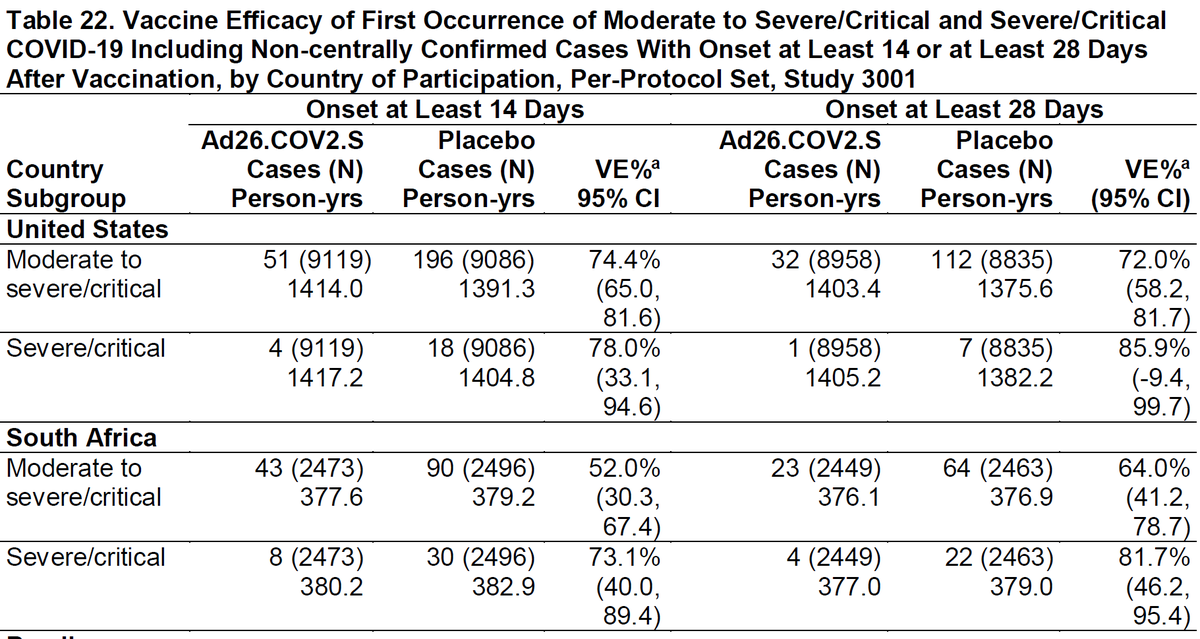
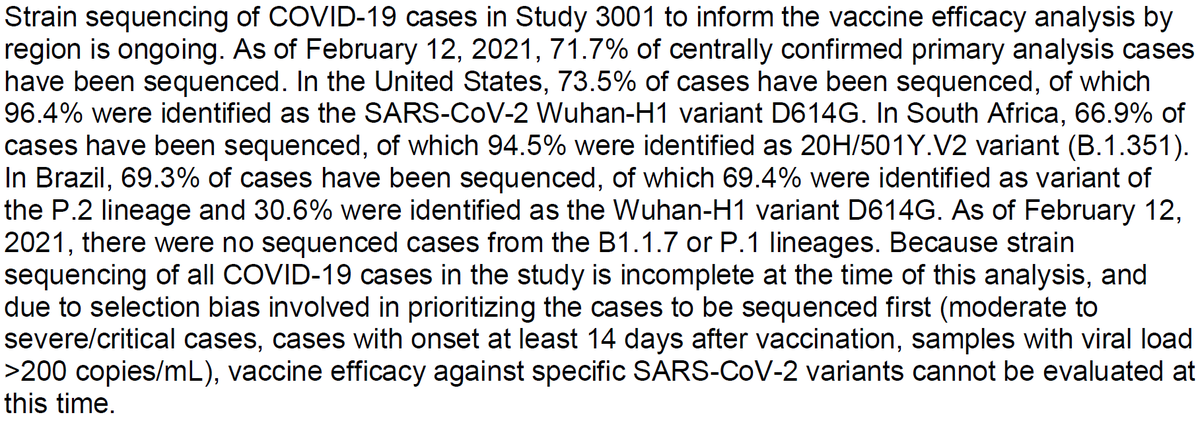
Interesting thread @trvrb on growth of B.1.1.7, P.1, B.1.351 in US
I worry about key assumptions underlying these analyses:
-sequences are random sample of state/US infections (definitely not)
-trends in sequences represent local transmission rather than changes in imported cases
I worry about key assumptions underlying these analyses:
-sequences are random sample of state/US infections (definitely not)
-trends in sequences represent local transmission rather than changes in imported cases
https://twitter.com/trvrb/status/1389248436071919628
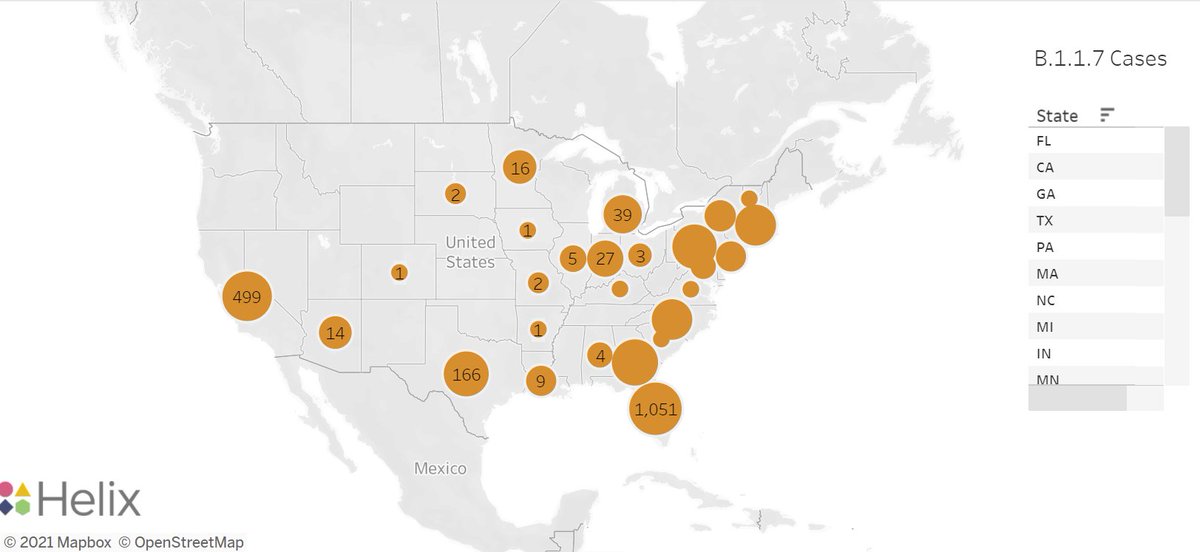
Many of the P.1, B.1.351 numbers are very low & introduced cases can make a substantial contribution to total count. Increased transmission elsewhere (e.g. Europe, S America) & constant introductions can lead to apparent increase in US for foreign variants.
In addition, increased detection of introduced cases can also bias results towards observing a local increase of an introduced variant.
Are cases (not frequencies) of P.1 really increasing in many states as @trvrb suggests? Possibly.
Are cases (not frequencies) of P.1 really increasing in many states as @trvrb suggests? Possibly.
But I'd be more convinced by analyses that address issues described above.
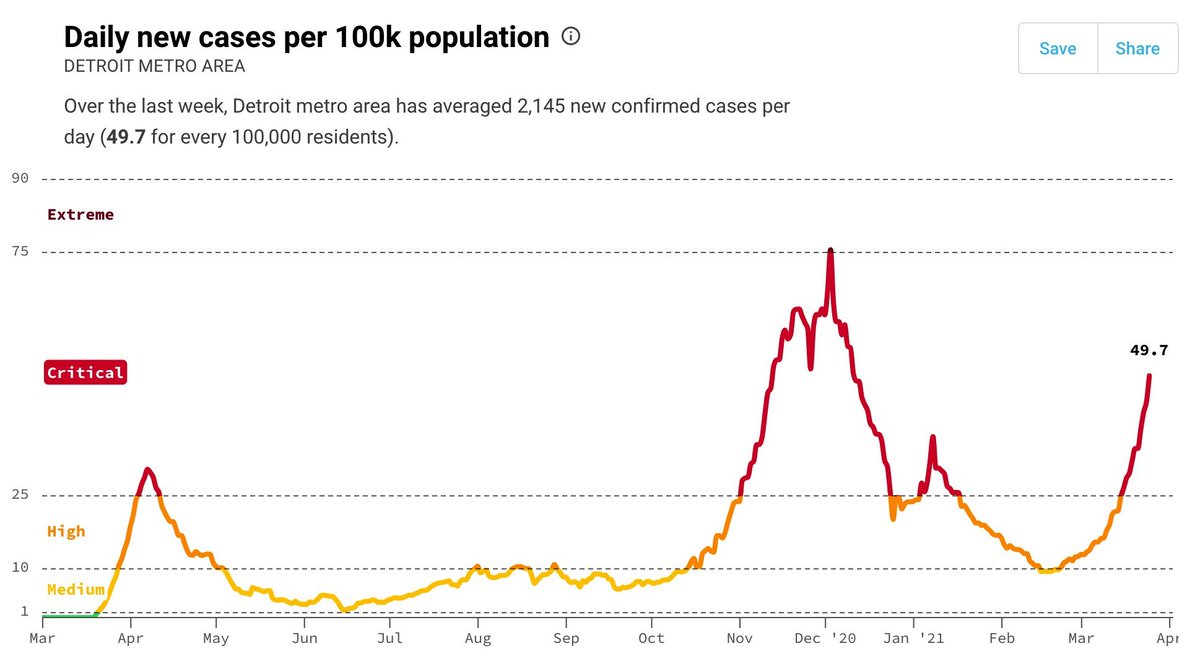
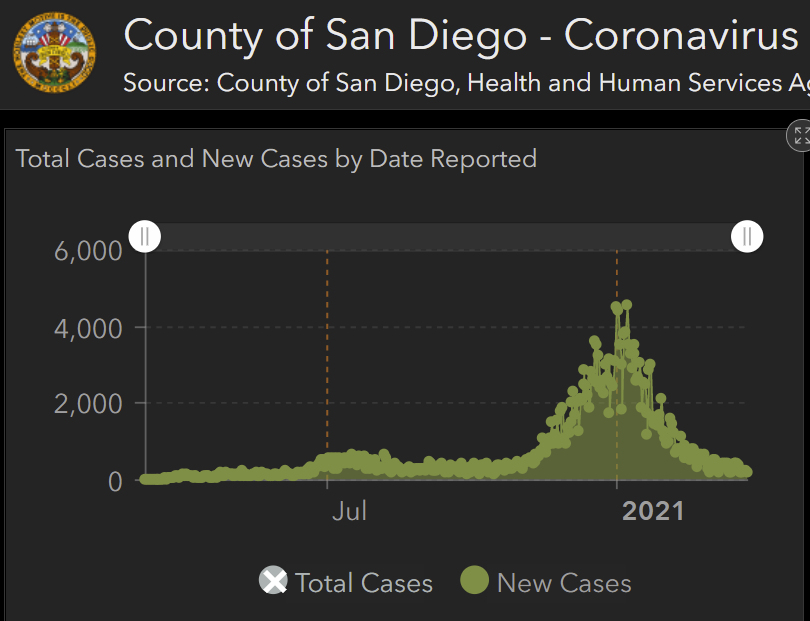
Locally, where overall R is <1, we've observed several variants inc. B.1.1.7 being introduced, leading to chains of transmission and then fading out. Summed across a state, this could appear to be growth.
Locally, where overall R is <1, we've observed several variants inc. B.1.1.7 being introduced, leading to chains of transmission and then fading out. Summed across a state, this could appear to be growth.
Instead, so far at least, these introductions have not established, and cases are now very low and falling. If vaccination continues, I believe P.1 & other variants can be suppressed.
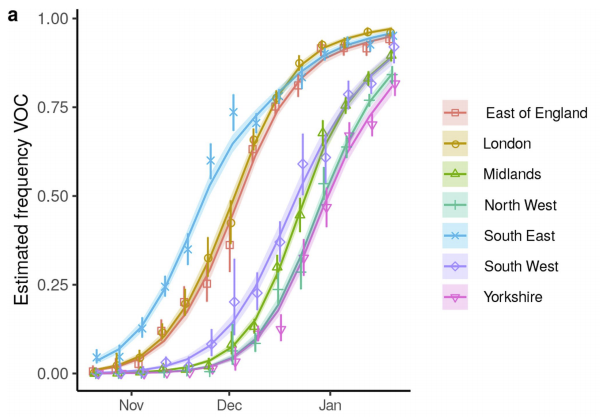
Obviously B.1.1.7 is being transmitted locally & contributed to surges in some/many states. But the extent of local transmission for P.1. is less clear to me at present. But our best insurance to stop spread of all variants is continued vaccination, as @trvrb suggests!
• • •
Missing some Tweet in this thread? You can try to
force a refresh