
We are extremely happy to present our lab’s first #preprint! Together with @teamstembryo we identify #hypoxia as a major microenvironmental factor that governs ES cell lineage commitment.
biorxiv.org/content/10.110…
Short summary 👇🏼👇🏼 (1/11)
biorxiv.org/content/10.110…
Short summary 👇🏼👇🏼 (1/11)
Have you ever wondered how environmental factors such as #hypoxia together with intrinsic cellular regulators shape stem cell identity and differentiation capacity? We have (a lot!). But wait a minute you say, isn’t oxygen essential for mammalian life? (2/11)
Of course it is! But… before placentation, the embryo develops in an increasingly hypoxic environment where oxygen tension is as low as 1.5%. But what’s the physiological relevance? @odedrechavi
(3/11)
(3/11)
Investigating the impact of hypoxia on embryonic & extraembryonic stem cells. Deregulated genes in response to hypoxia were associated with development & differentiation. Most exciting observation? Hypoxic ES cells display a transcriptional early primitive streak signature!(4/11) 

But what’s the mechanism? We show that HIF1a activation is sufficient to upregulate primitive streak genes but also that DNA demethylation and glycolysis integratively shape the transcriptional response at developmental genes. (5/11) 

And the functional relevance? To test this, we needed an in-vivo-like model of embryo development that allowed for easy tuning of the cellular microenvironment. Enter: #gastruloids @teamstembryo. We imaged and analyzed over 500 of these beasts to make it quantitative! (6/11)
The conventional gastruloid protocol involves exogenous WNT activation to induce symmetry breaking, axial elongation and self-organization of the body axes with polarized T expression in the posterior end. (7/11)
As hypoxia induces Wnt3 and T expression, would hypoxic #gastruloids elongate in the absence of exogenous WNT activation? You bet! ~30% of the hypoxic structures show localized expression of T & do spontaneously elongate w/o any exogenous Wnt activation! #InNumbersWeTrust. (8/11) 
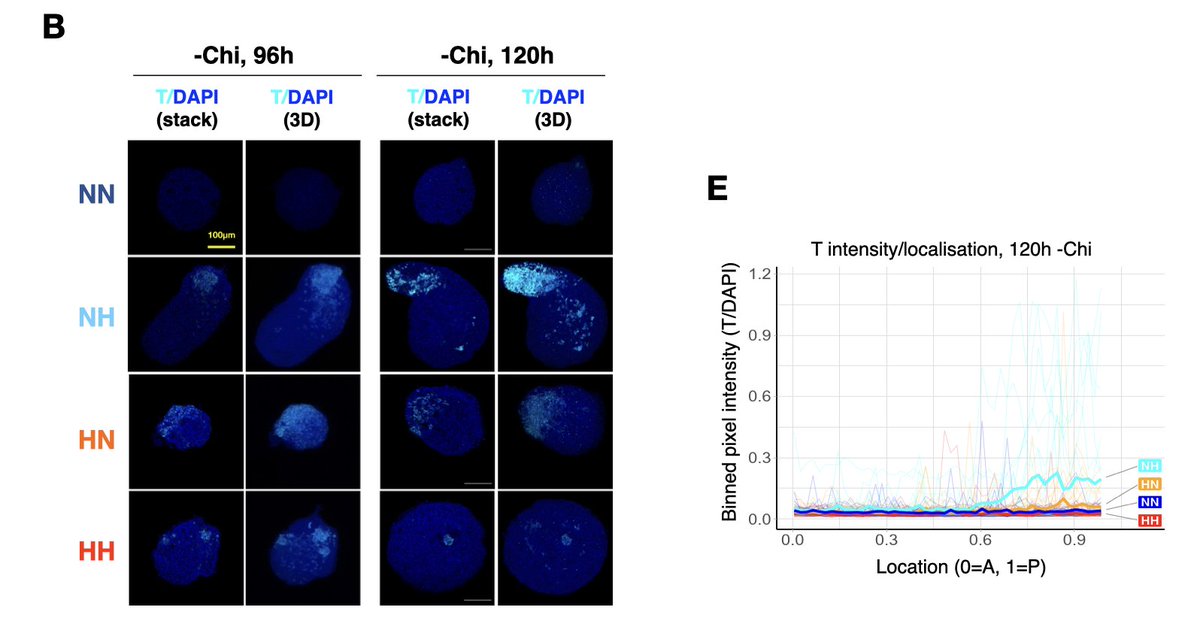
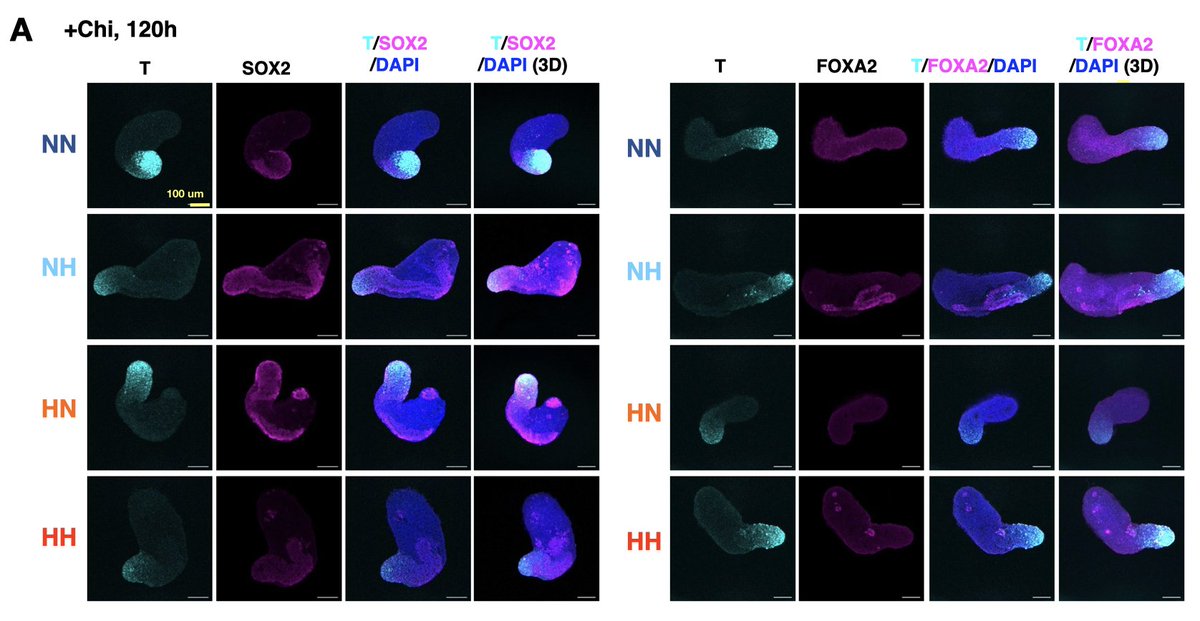
Finally, we tested how hypoxia impacts lineage representation in conventional gastruloids with exogenous Wnt activation. Turns out hypoxia modulates T and SOX2 expression and increases expression of paraxial mesoderm and #somite markers! (9/11) 
In conclusion, our findings show that hypoxia rewires the transcriptional, epigenetic and metabolic landscapes of stem cells, and that oxygen tension should be taken into consideration when modelling development in a dish, or culturing mammalian embryos ex utero. (10/11)
Last but not least, this work would not have been possible without colleagues at the @MPI_MolGen, in particular @IpekGassa, @Maxistoe, @jvveenvliet (now at the @mpicbg @teamstembryo) and mastermind @bulutkarslioglu. (11/11)
• • •
Missing some Tweet in this thread? You can try to
force a refresh



