Salvage therapy for prostate cancer after prostatectomy: international consensus on evaluation and management
@NatRevUrol
rdcu.be/csM2z
pubmed.ncbi.nlm.nih.gov/34363040/
#PCSM

@NatRevUrol
rdcu.be/csM2z
pubmed.ncbi.nlm.nih.gov/34363040/
#PCSM


Since the 2000s, the use of radical prostatectomy has been increasing for prostate cancer (vs external beam and brachytherapy).
@EUplatinum
pubmed.ncbi.nlm.nih.gov/27597241/
@EUplatinum
pubmed.ncbi.nlm.nih.gov/27597241/

The increase in prostatectomy includes all risk groups, particularly those with high-risk features
https://twitter.com/nicholaszaorsky/status/1204505104562491392
Many of these men have unfavorable pathologic features, eg pT3+ pN+, GG4+; or a persistently elevated PSA.
Use of post-operative adjuvant therapy remains low, <5%.
pubmed.ncbi.nlm.nih.gov/33128858/
Use of post-operative adjuvant therapy remains low, <5%.
pubmed.ncbi.nlm.nih.gov/33128858/

Subsequently, ~40% men have biochemically recurrent disease (a rising PSA) after the surgery. In the US, >30K men are diagnosed with biochemically recurrent dz / year, enough to fill @fenwaypark 

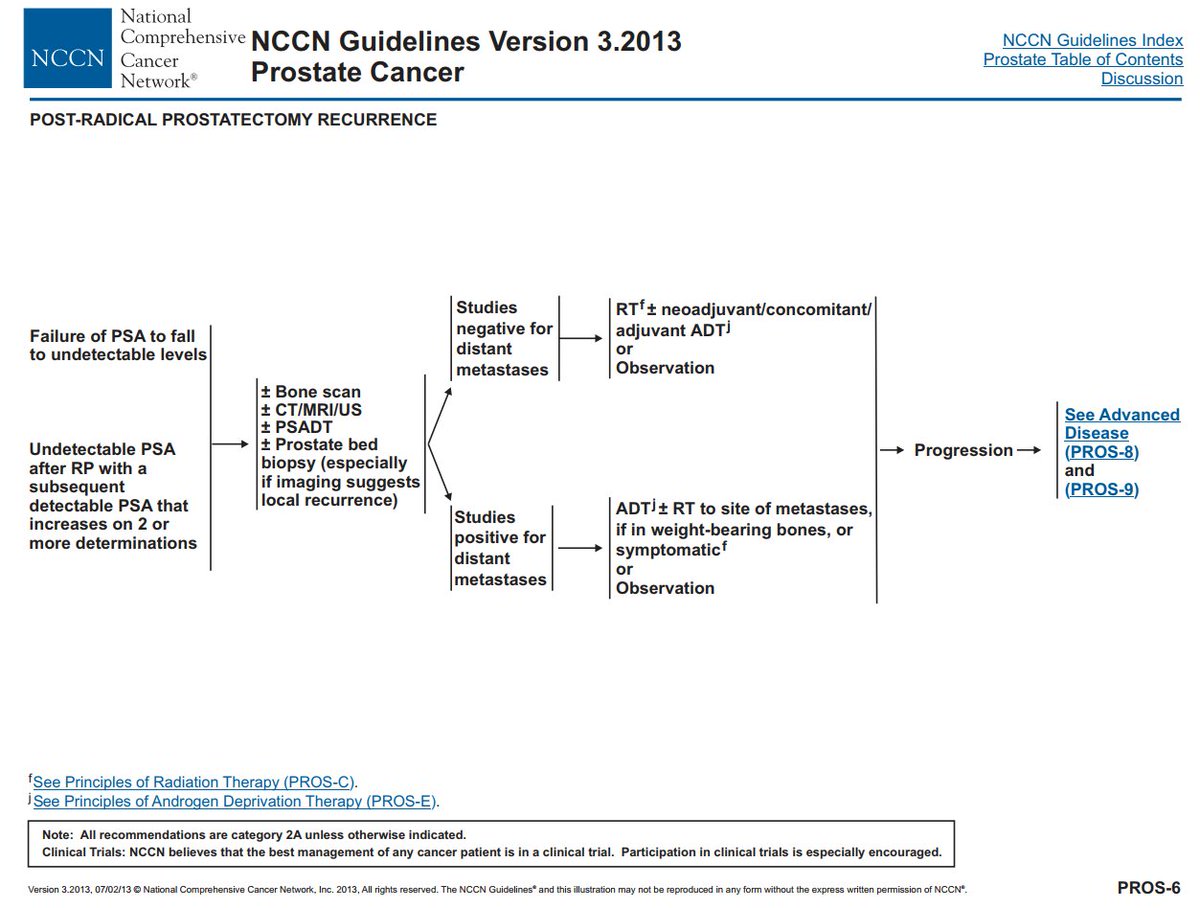
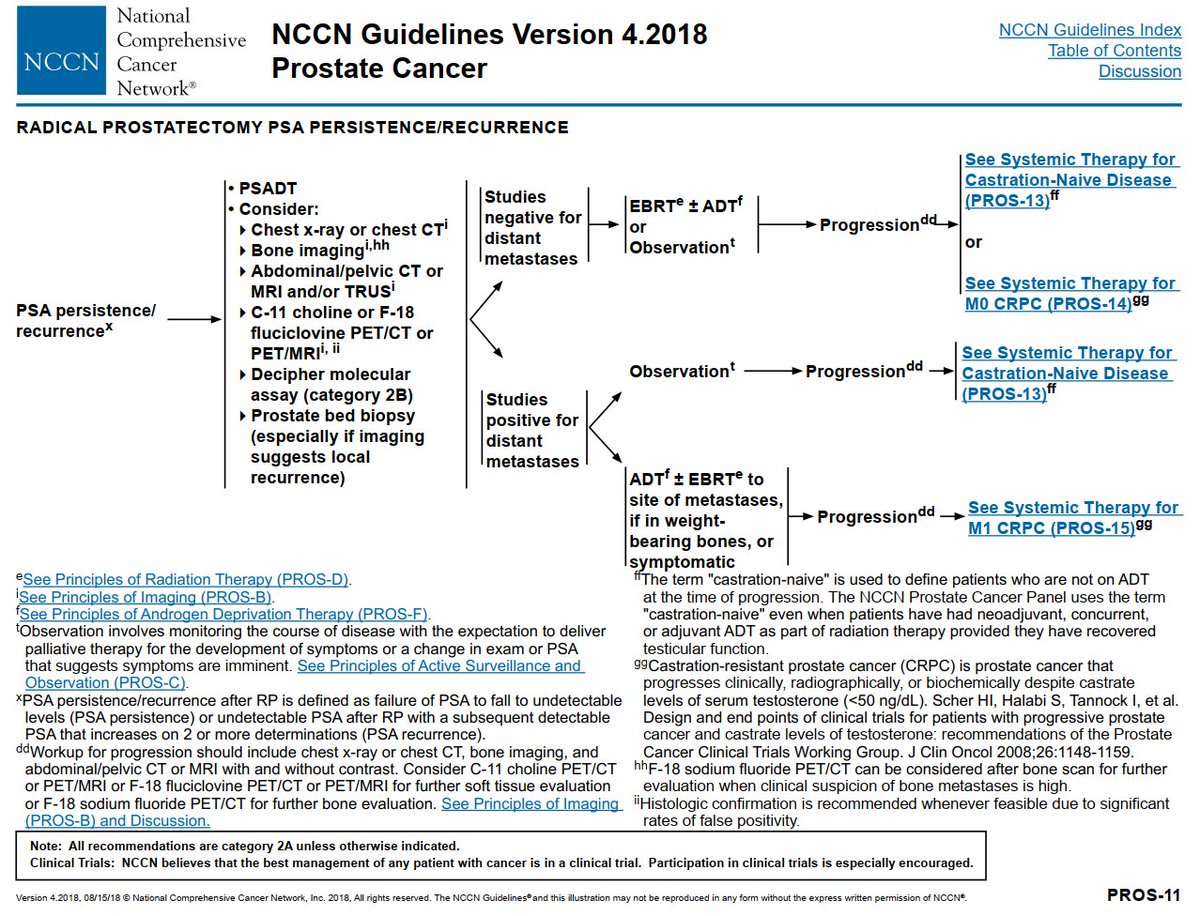
The guidelines for PSA recurrences have been evolving, eg @NCCN 2013 vs 2021.
The overall schema: does the patients have metastases?
If yes, then systemic therapy. If no, then local therapy +/- hormones; or observe. @Uroweb @AmerUrological guidelines similar.

The overall schema: does the patients have metastases?
If yes, then systemic therapy. If no, then local therapy +/- hormones; or observe. @Uroweb @AmerUrological guidelines similar.
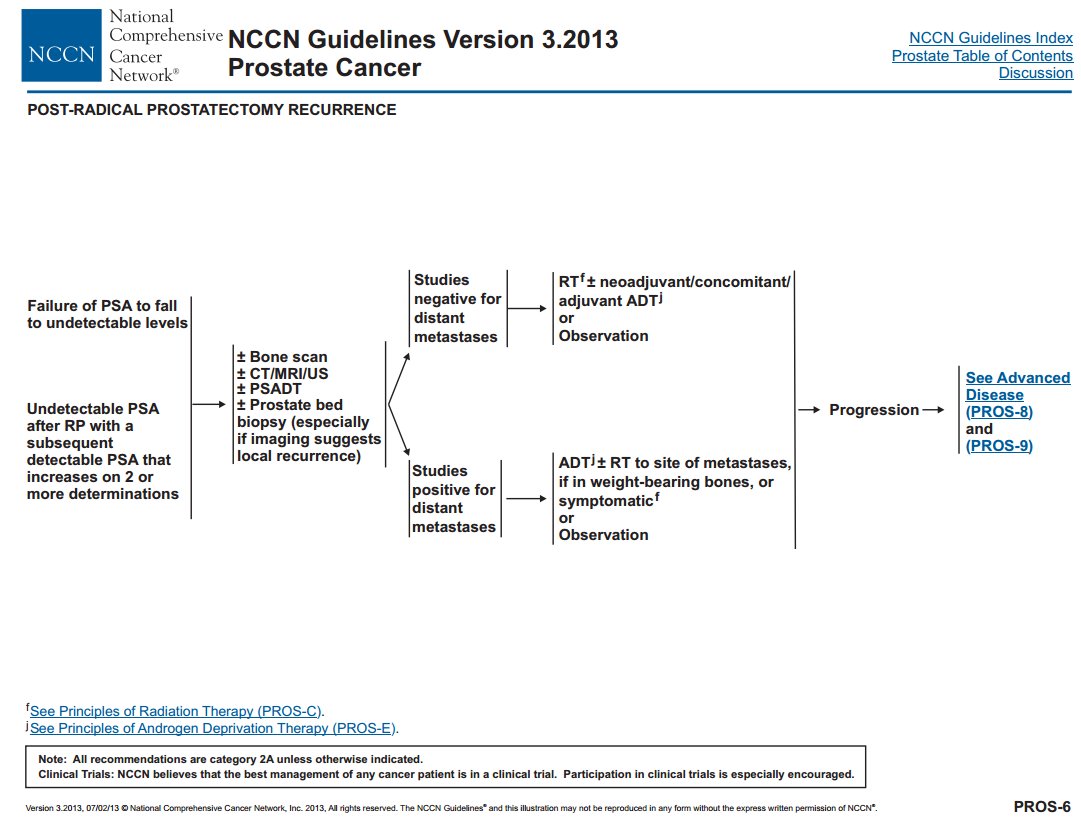

We provide this review to help providers and pts.
What is a biochemical recurrence? The definition is different after prostatectomy vs radiation. After prostatectomy, PSA should be undetectable, historically <0.2 ng/mL, though newer tests more sensitive.
pubmed.ncbi.nlm.nih.gov/28664931/

What is a biochemical recurrence? The definition is different after prostatectomy vs radiation. After prostatectomy, PSA should be undetectable, historically <0.2 ng/mL, though newer tests more sensitive.
pubmed.ncbi.nlm.nih.gov/28664931/
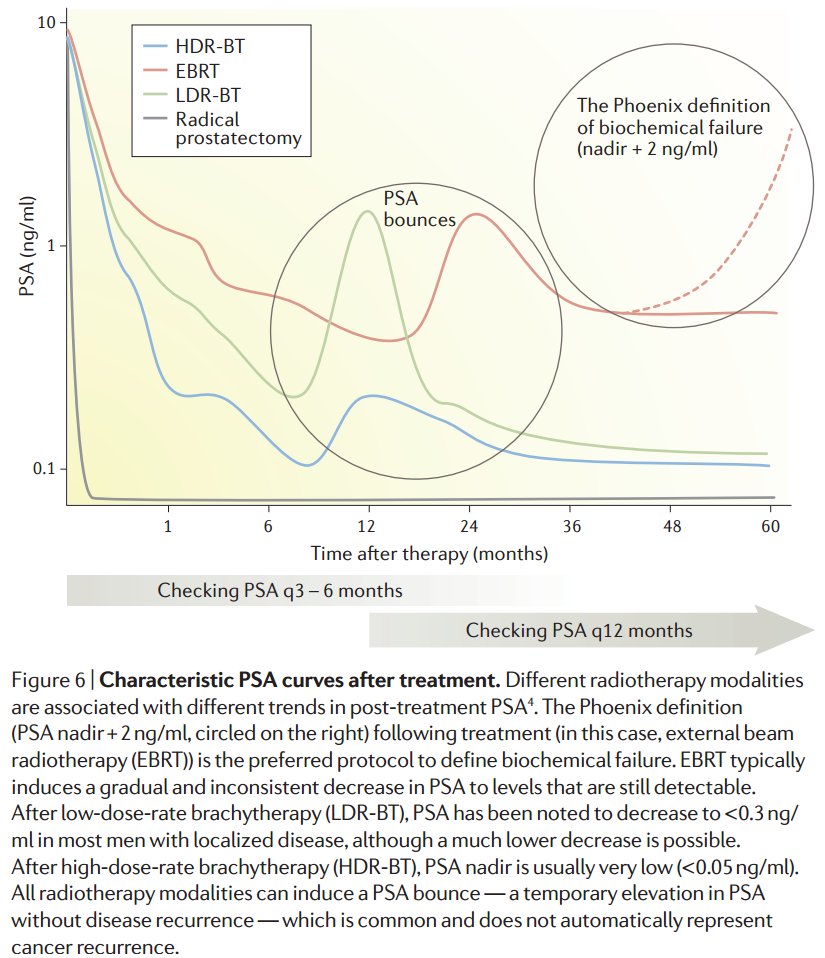

PSA kinetics are key:
Persistently elevated post op PSA implies metastases.
Short doubling time (eg, <8-10 mos) implies fast dissemination.
@Tilki_De
pubmed.ncbi.nlm.nih.gov/33574012/
@RealProstateDoc
pubmed.ncbi.nlm.nih.gov/17513807/

Persistently elevated post op PSA implies metastases.
Short doubling time (eg, <8-10 mos) implies fast dissemination.
@Tilki_De
pubmed.ncbi.nlm.nih.gov/33574012/
@RealProstateDoc
pubmed.ncbi.nlm.nih.gov/17513807/


A few drugs impact interpretation:
pre-op ADT ➡️ post-op PSA = 0
post-op 5a reductase inhibitors (eg, finasteride) = longer doubling time
Though usually not considered, aspirin, statin, HCTZ also ↘️ PSA. Important if considering certain cut points (eg 0.5 ng/mL to add ADT)
pre-op ADT ➡️ post-op PSA = 0
post-op 5a reductase inhibitors (eg, finasteride) = longer doubling time
Though usually not considered, aspirin, statin, HCTZ also ↘️ PSA. Important if considering certain cut points (eg 0.5 ng/mL to add ADT)
Predicting cause of death is difficult. Presumably, these men were healthy enough for surgery, so most will die of competing causes >> prostate cancer.
Our work, still under way...
@DrSpratticus @AmarUKishan

Our work, still under way...
@DrSpratticus @AmarUKishan


Our group has been predicting risk of death from the main competing causes (via @NatureComms):
heart disease:
stroke:
suicide:
heart disease:
https://twitter.com/NicholasZaorsky/status/1254518239243243522
stroke:
https://twitter.com/NicholasZaorsky/status/1195700095037136898
suicide:
https://twitter.com/NicholasZaorsky/status/1084788409338683394
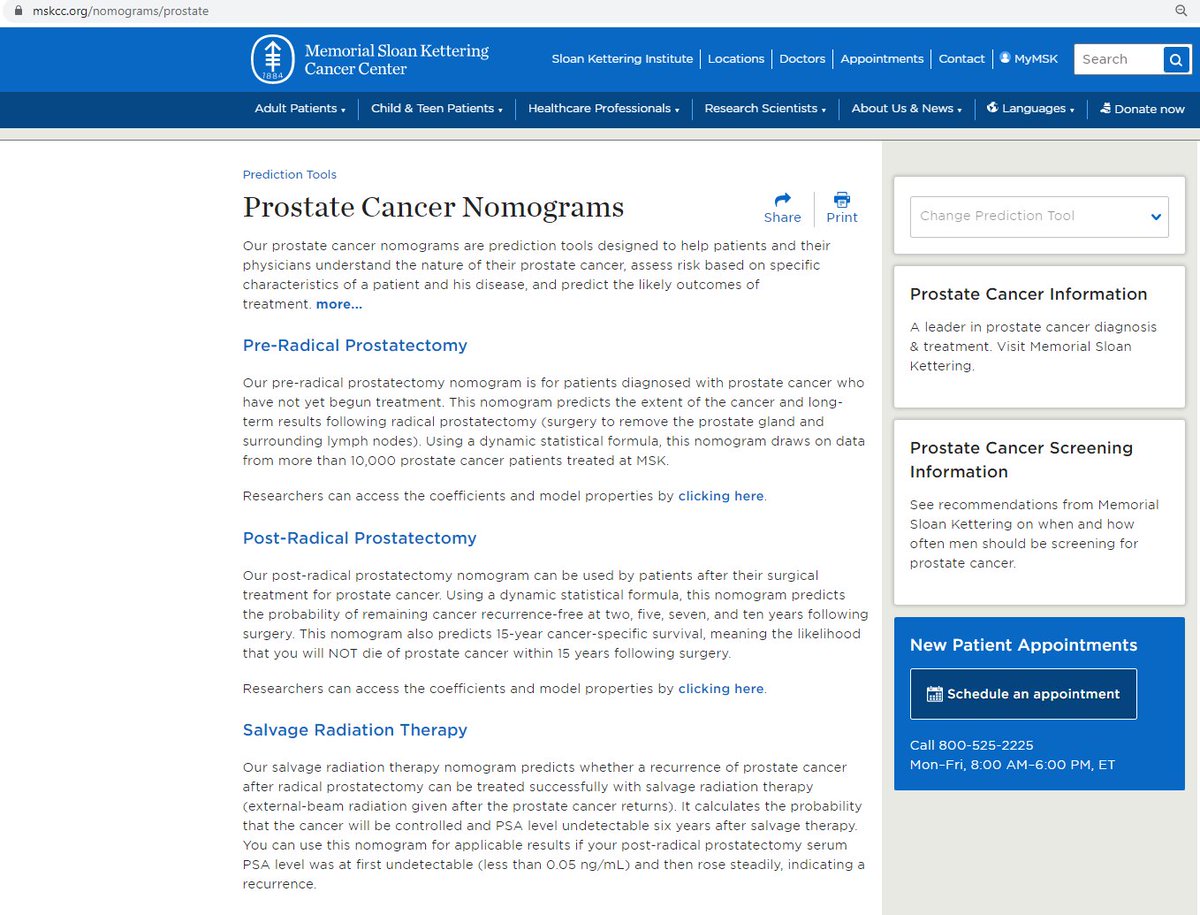
The nomograms from @sloan_kettering are useful to predict risk of death from prostate cancer
mskcc.org/nomograms/pros…
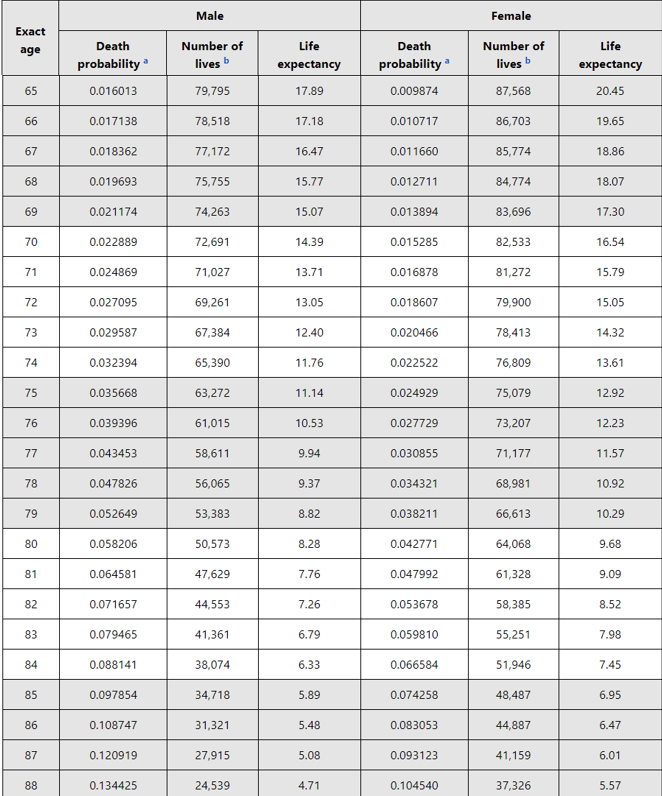
@NCCN also recommends using social security life expectancy tables: ssa.gov/OACT/STATS/tab…

mskcc.org/nomograms/pros…
@NCCN also recommends using social security life expectancy tables: ssa.gov/OACT/STATS/tab…
Next, on to imaging.
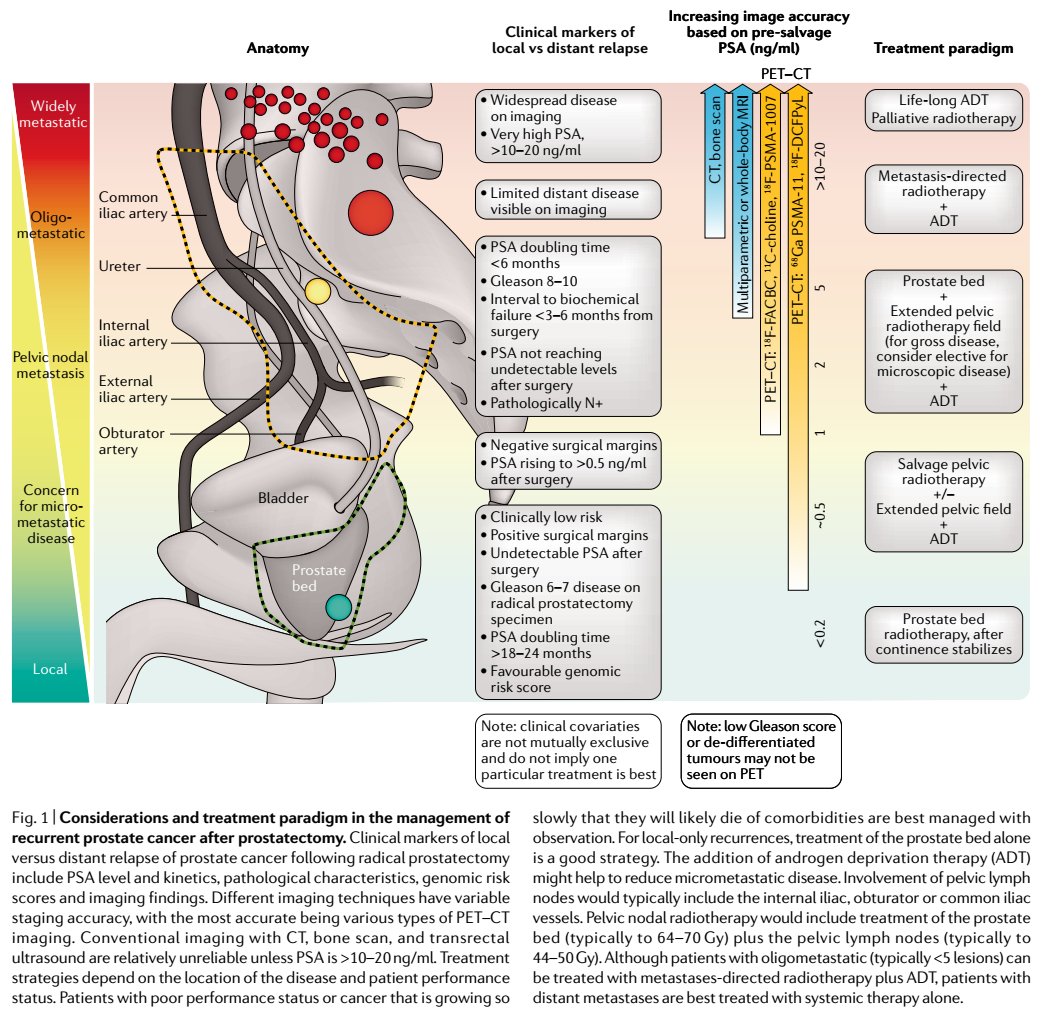
For majority of patients in US, the main scans available are CT, bone scan, pelvic MRI.
However, their accuracy is limited; we don't recommend CT or bone scan for PSA < 5 ng/mL.
For majority of patients in US, the main scans available are CT, bone scan, pelvic MRI.
However, their accuracy is limited; we don't recommend CT or bone scan for PSA < 5 ng/mL.

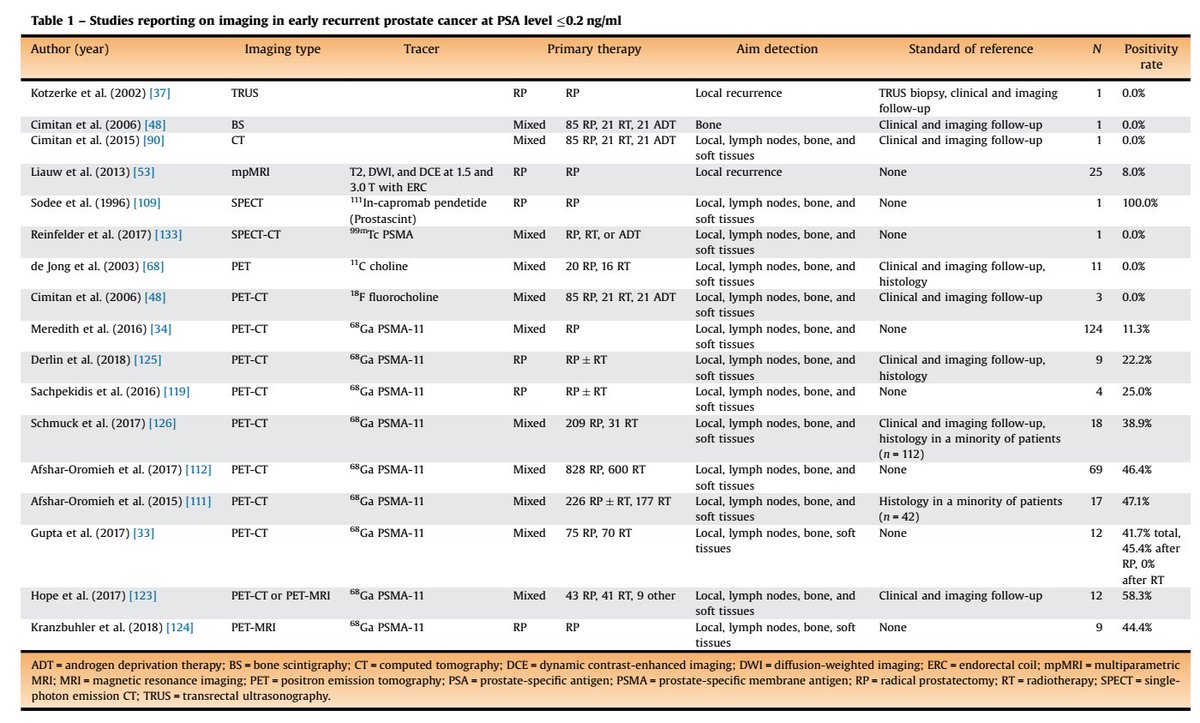
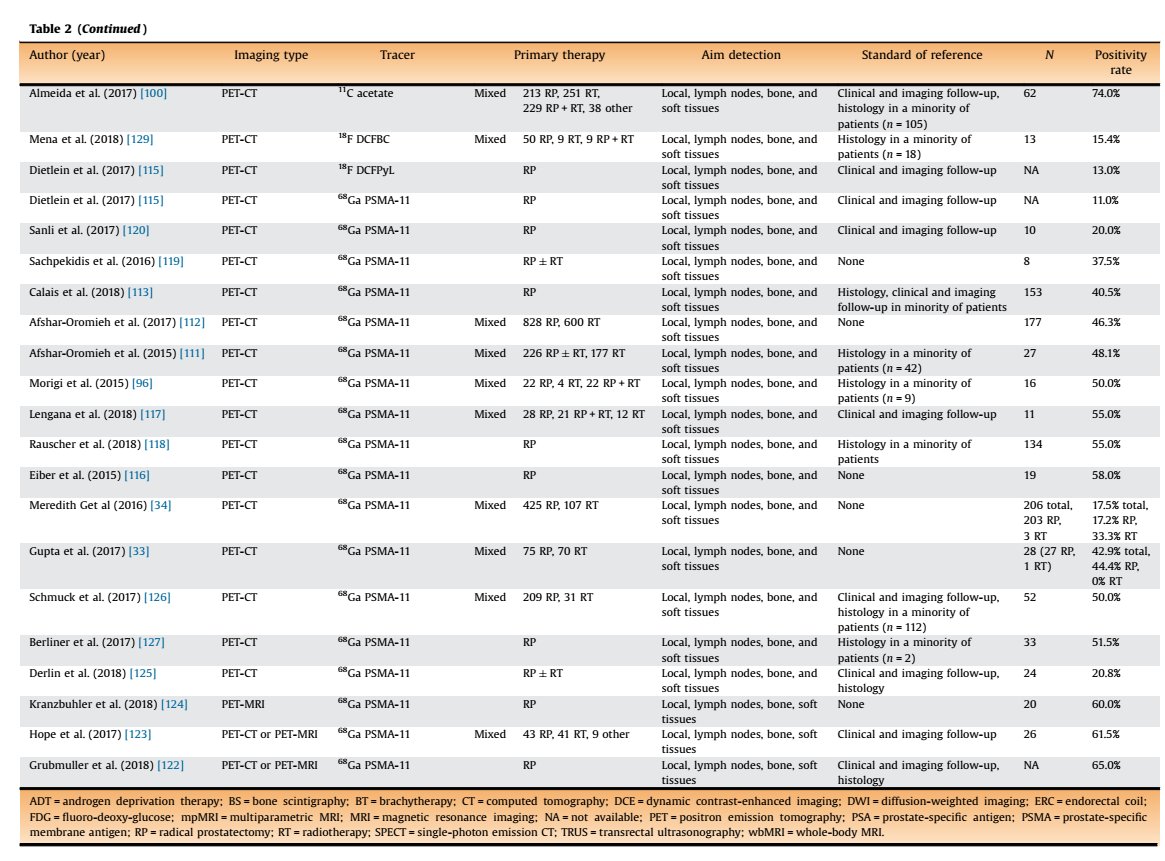
Here is an excellent systematic review on the role of imaging in early recurrent prostate ca.
Table 1 is for PSA < 0.2
Table 2 is for PSA < 0.5
pubmed.ncbi.nlm.nih.gov/30929846/
@stefanofanti4 @GGiannarini @EurUrolOncol


Table 1 is for PSA < 0.2
Table 2 is for PSA < 0.5
pubmed.ncbi.nlm.nih.gov/30929846/
@stefanofanti4 @GGiannarini @EurUrolOncol

The most popular PET-CT options in the salvage setting include fluciclovine (Axumin) and PSMA.
Comprehensive review from Luca Valle @AmarUKishan @CalaisJeremie @NehaVapiwala @GertMeerleer @declangmurphy
pubmed.ncbi.nlm.nih.gov/33637464/

Comprehensive review from Luca Valle @AmarUKishan @CalaisJeremie @NehaVapiwala @GertMeerleer @declangmurphy
pubmed.ncbi.nlm.nih.gov/33637464/
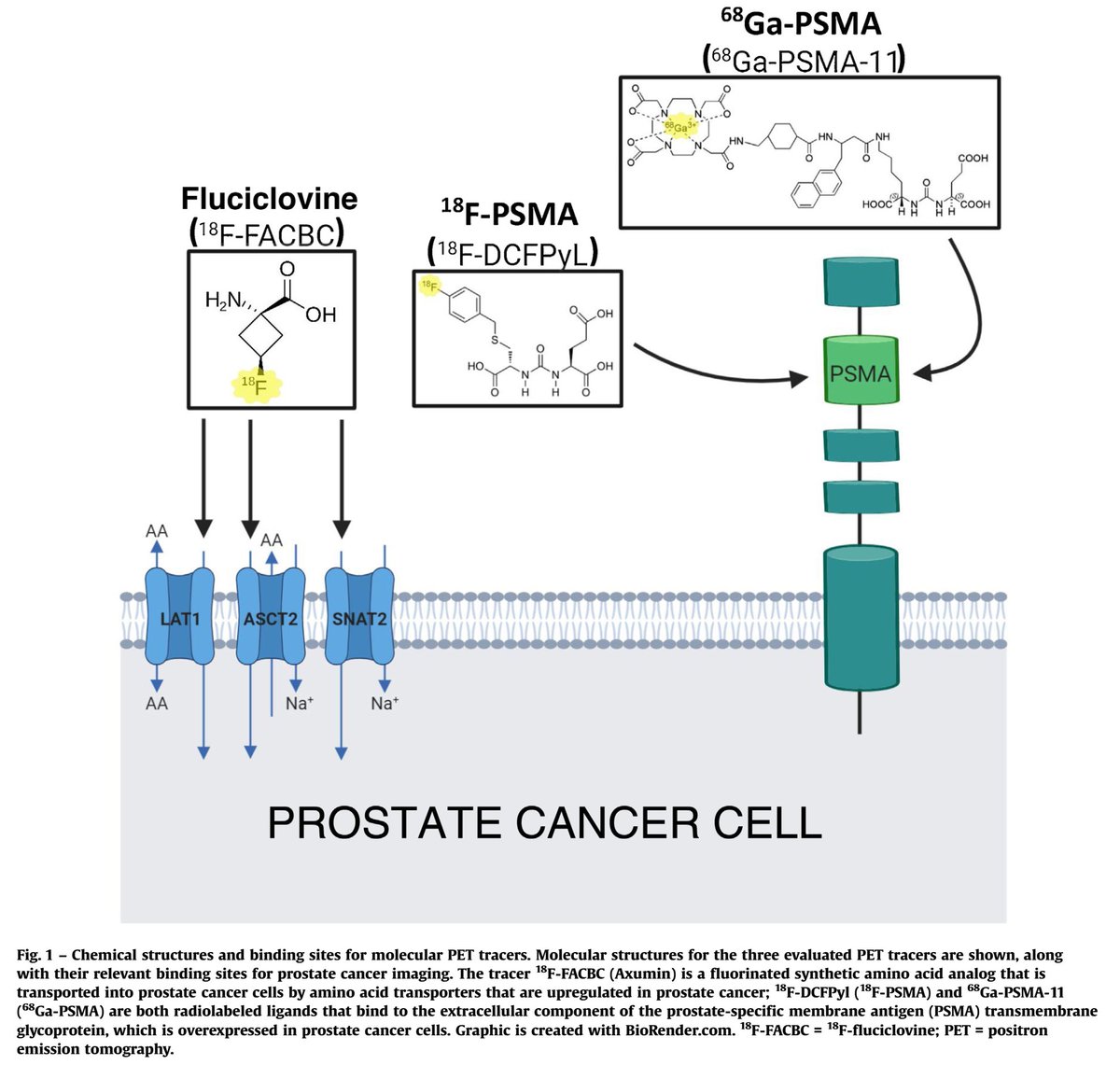
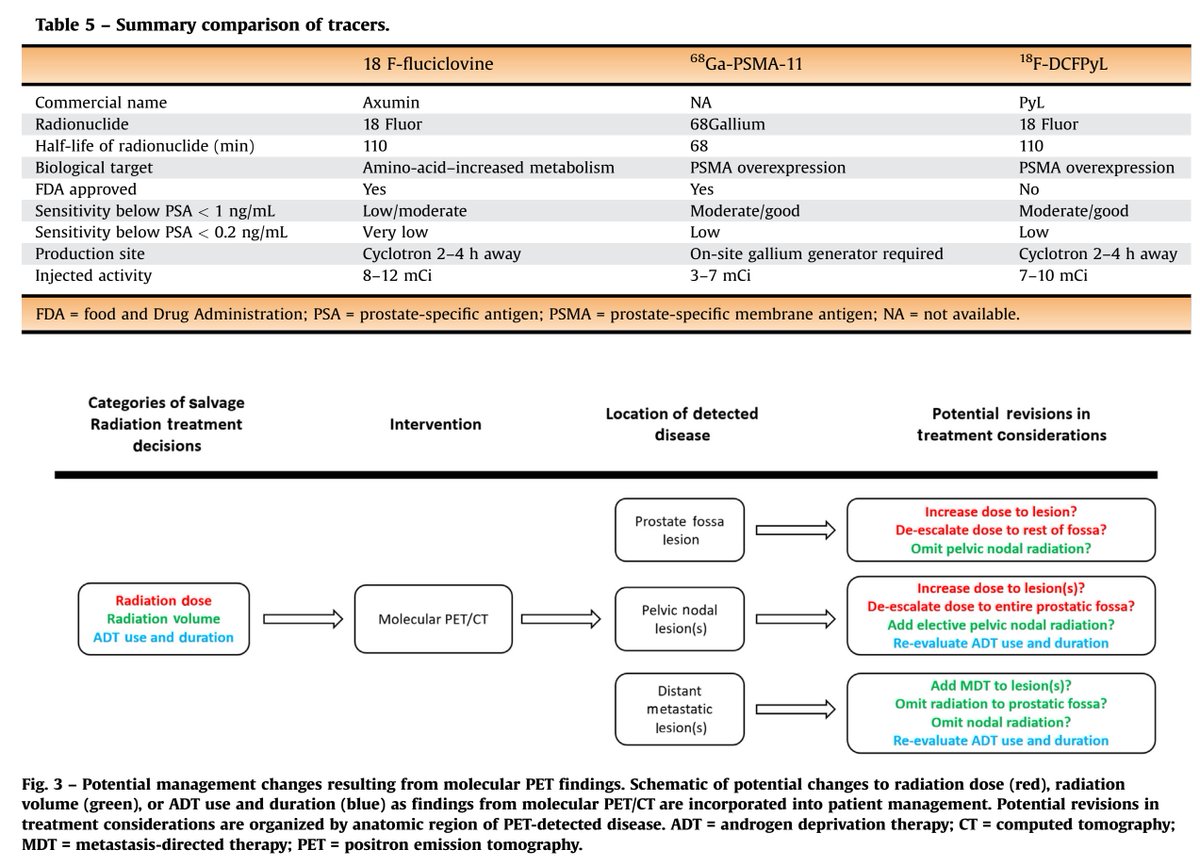
Accuracy of fluciclovine PET is limited with PSA < 1-2 ng/mL. A cut point of adding ADT to RT (a major treatment decision) for a biochemical recurrence is usually ~0.5-0.7 ng/mL.
Thus, most men with a slowly rising PSA do not benefit from the scan.
Thus, most men with a slowly rising PSA do not benefit from the scan.
MRIs, similarly, typically do not show local recurrence with such low PSAs. However, they are useful to identify the vesicourethral anastomosis (junction of bladder and urethra), and this is used in radiation therapy planning. 

On the other end of the spectrum, for pt w rapidly rising PSA to 10-100s ng/mL, metastasis is real concern. Prostate ca preferentially metastasizes to the bone (> liver, lung, brain) and should be on top of Ddx for new bone lesions.
pubmed.ncbi.nlm.nih.gov/33276151/
@skhosla78 @CPSolvers
pubmed.ncbi.nlm.nih.gov/33276151/
@skhosla78 @CPSolvers

If you were to use 68Ga-PSMA-11 PET to map prostate cancer biochemical recurrence with PSA < 1 ng/mL, it would look like this
pubmed.ncbi.nlm.nih.gov/29123013/

pubmed.ncbi.nlm.nih.gov/29123013/


A PSMA-PET-based lymph node atlas as been developed to help guide salvage radiotherapy.
@SE_Combs @EurUrolOncol
pubmed.ncbi.nlm.nih.gov/32451312/



@SE_Combs @EurUrolOncol
pubmed.ncbi.nlm.nih.gov/32451312/

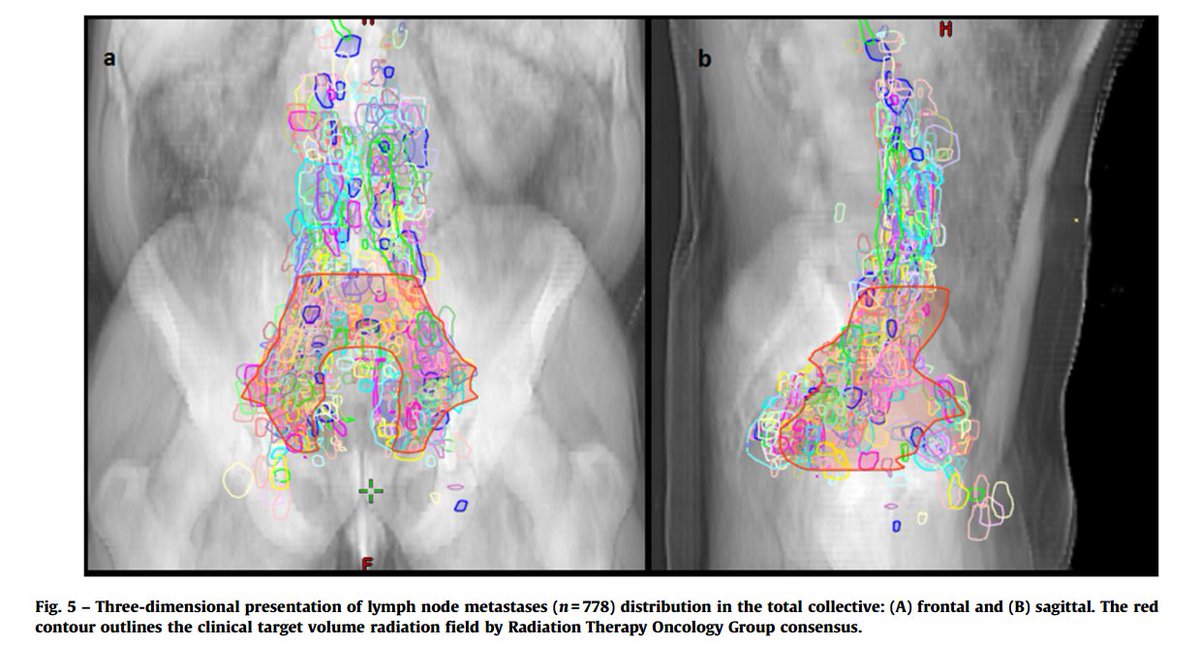
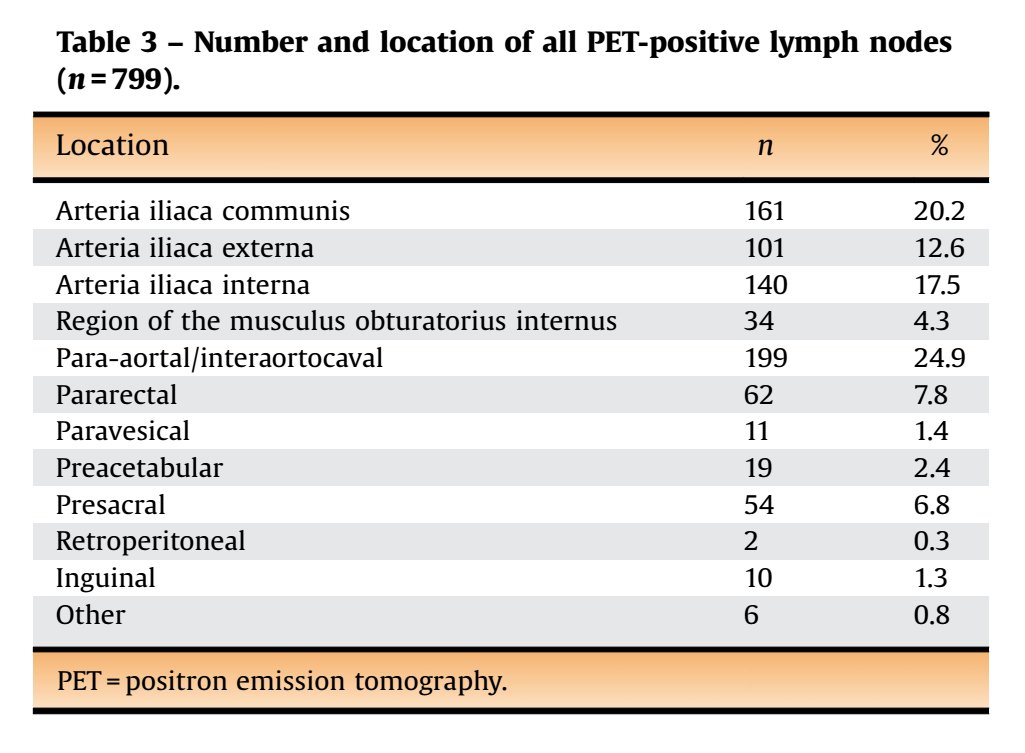

Similarly, 11C-choline PET maps recurrence.
Notably, the median PSA for local only, metastatic only, or combined recurrence was 2.3, 2.7, 2.2 ng/mL (all similar).
pubmed.ncbi.nlm.nih.gov/27449262/
Limitations of 11C: 20 min half life (cyclotron needed), accuracy limited at < 1-2 ng/mL


Notably, the median PSA for local only, metastatic only, or combined recurrence was 2.3, 2.7, 2.2 ng/mL (all similar).
pubmed.ncbi.nlm.nih.gov/27449262/
Limitations of 11C: 20 min half life (cyclotron needed), accuracy limited at < 1-2 ng/mL


Historically, the main scans radiation oncologists used for treatment planning were CT and MRI.
In 2010, the @ASTRO_org @NRGonc post-prostatectomy contouring atlas was published, based on composite contours.
pubmed.ncbi.nlm.nih.gov/19394158/



In 2010, the @ASTRO_org @NRGonc post-prostatectomy contouring atlas was published, based on composite contours.
pubmed.ncbi.nlm.nih.gov/19394158/


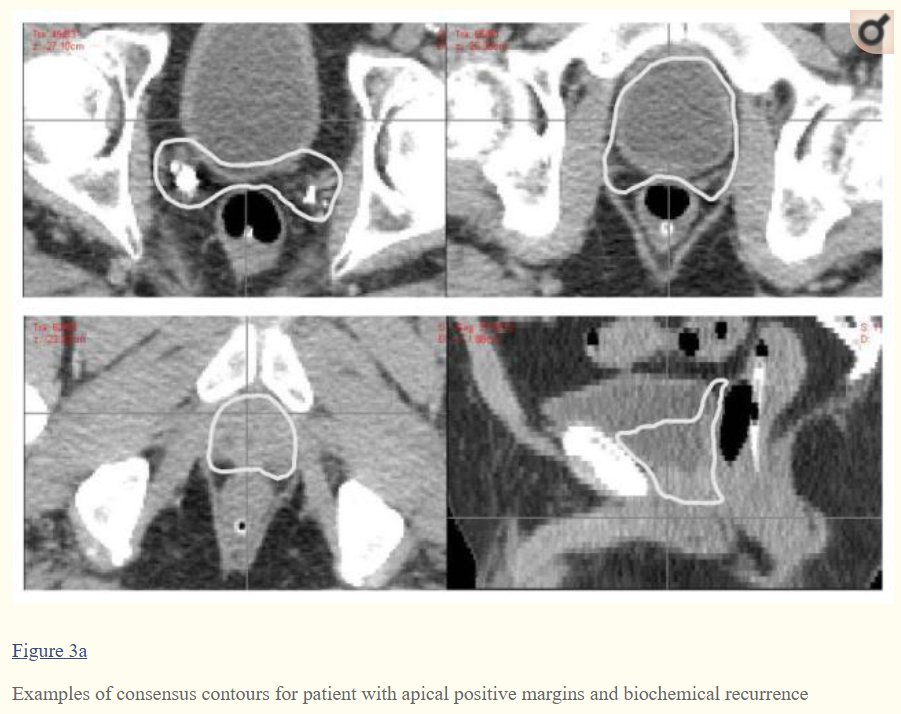

Here are sites of local recurrence on MRI vs the
@NRGonc @ASTRO_org #RadOnc consensus contours.
pubmed.ncbi.nlm.nih.gov/25407875/


@NRGonc @ASTRO_org #RadOnc consensus contours.
pubmed.ncbi.nlm.nih.gov/25407875/



An @NRGonc @ASTRO_org #RadOnc contouring atlas has also been made for the pelvic lymph nodes.
pubmed.ncbi.nlm.nih.gov/32861817/
@whallradonc
One could add this volume on to the prostate bed volume, if needed.
The prostate bed usually receives ~66-70 Gy. The nodes receive ~45-50 Gy.

pubmed.ncbi.nlm.nih.gov/32861817/
@whallradonc
One could add this volume on to the prostate bed volume, if needed.
The prostate bed usually receives ~66-70 Gy. The nodes receive ~45-50 Gy.


Physicians in #RadOnc today are lucky to have resources like @eContourRadOnc, which puts info on the target volumes, organs at risk, margins, and references all in one site.
econtour.org/cases
@ARRO_org
econtour.org/cases
@ARRO_org

So far, we have reviewed epidemiology, workup, imaging. Next, treatment.
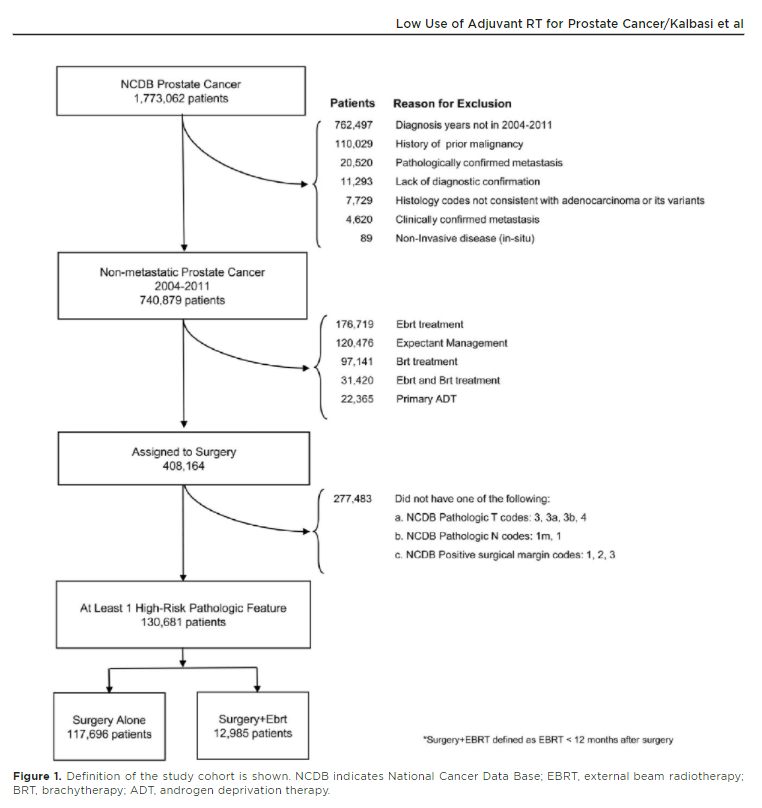
If a radiation oncologist were to treat every patient with high risk pathologic features (eg, pT3+, pN1, R1), about 1/3 patients would be treated with adjuvant RT.
acsjournals.onlinelibrary.wiley.com/doi/epdf/10.10…
@JournalCancer

If a radiation oncologist were to treat every patient with high risk pathologic features (eg, pT3+, pN1, R1), about 1/3 patients would be treated with adjuvant RT.
acsjournals.onlinelibrary.wiley.com/doi/epdf/10.10…
@JournalCancer
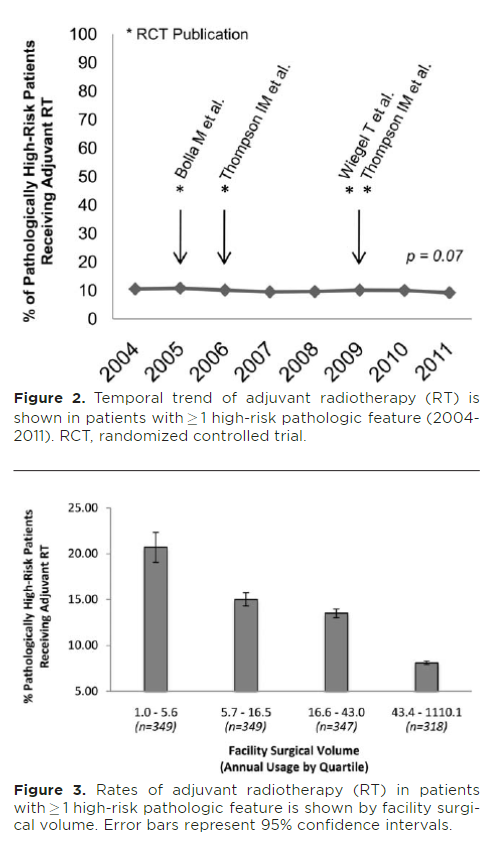
There were several studies done in 80s-00s evaluating role of adjuvant RT (undetectable PSA, hi risk features). Rationale made sense: RT indicated for similar features in other cancers. There was improvement in PSA free survival.
pubmed.ncbi.nlm.nih.gov/23084481/
pubmed.ncbi.nlm.nih.gov/19433689/



pubmed.ncbi.nlm.nih.gov/23084481/
pubmed.ncbi.nlm.nih.gov/19433689/

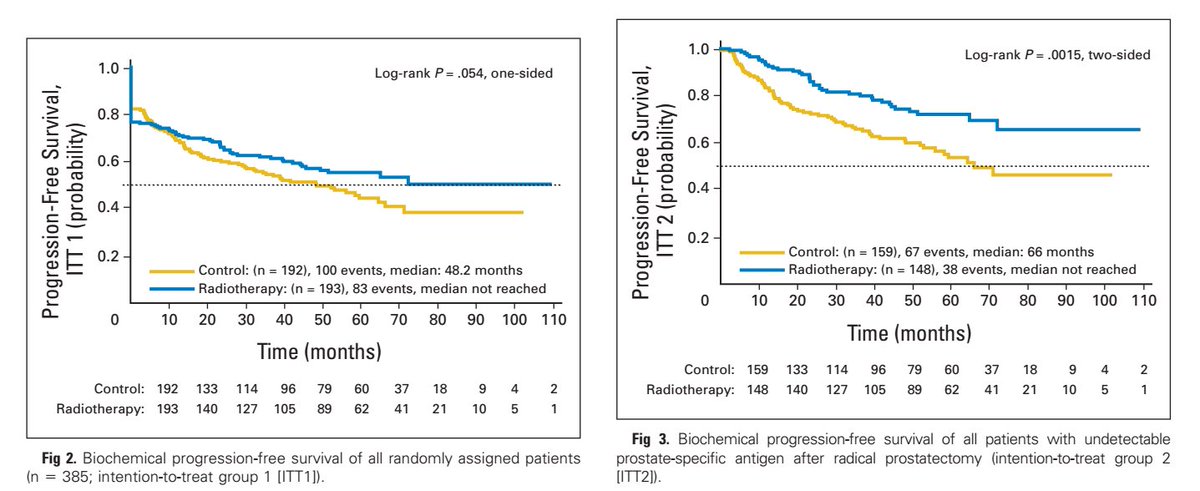


SWOG 8794 even showed improvement in distant metastasis free survival and overall survival.
Boards mnemonic: "The S in SWOG is for survival."
auajournals.org/doi/10.1016/j.…


Boards mnemonic: "The S in SWOG is for survival."
auajournals.org/doi/10.1016/j.…



• • •
Missing some Tweet in this thread? You can try to
force a refresh