
COPTIGATE - THE WORST DESIGN FLAW IN HUMAN HISTORY THAT IS IMPACTING YOUR HEALTH.
(thread)
How come Prizer, Moderna, AstraZeneca, Janssen etc. are using a technology that both they and the regulators know will cause unknown results?
(plus a bonus)
#COptiGate
(thread)
How come Prizer, Moderna, AstraZeneca, Janssen etc. are using a technology that both they and the regulators know will cause unknown results?
(plus a bonus)
#COptiGate

Let's start with a thought experiment:
If an engineering design flaw exists and no one measures it, can it really injure people or kill them?
#COptiGate
If an engineering design flaw exists and no one measures it, can it really injure people or kill them?
#COptiGate
Making a new vaccine is hard. Making a new vaccine that uses a new technology is even harder, because you need to prove safety.
Luckily, when it comes to COVID, the vaccines have been tested and shown to be safe, right?
Well, they might have forgotten one thing...
#COptiGate
Luckily, when it comes to COVID, the vaccines have been tested and shown to be safe, right?
Well, they might have forgotten one thing...
#COptiGate
Trying to tell your body to generate proteins is hard for many reasons. One of them is the fact that when you try to run the protein information via ribosomes which process that code and generate the protein, it can be very slow or can get stuck during the process.
#COptiGate
#COptiGate

Luckily, scientists found a way to overcome this problem, by doing code substitution: instead of using the original genetic code to generate the protein, they changed the letters in the code so the code would be optimized. This is known as Codon Optimization.
#COptiGate
#COptiGate
Codons are three nucleotides; nucleotides are the building blocks of your DNA.
Here is an example of Codon Optimization:
60% of the codons were altered,
22% of the nucleotides were altered.
And yet the end result is that the ribosomes generate the same protein!
#COpiGate
Here is an example of Codon Optimization:
60% of the codons were altered,
22% of the nucleotides were altered.
And yet the end result is that the ribosomes generate the same protein!
#COpiGate
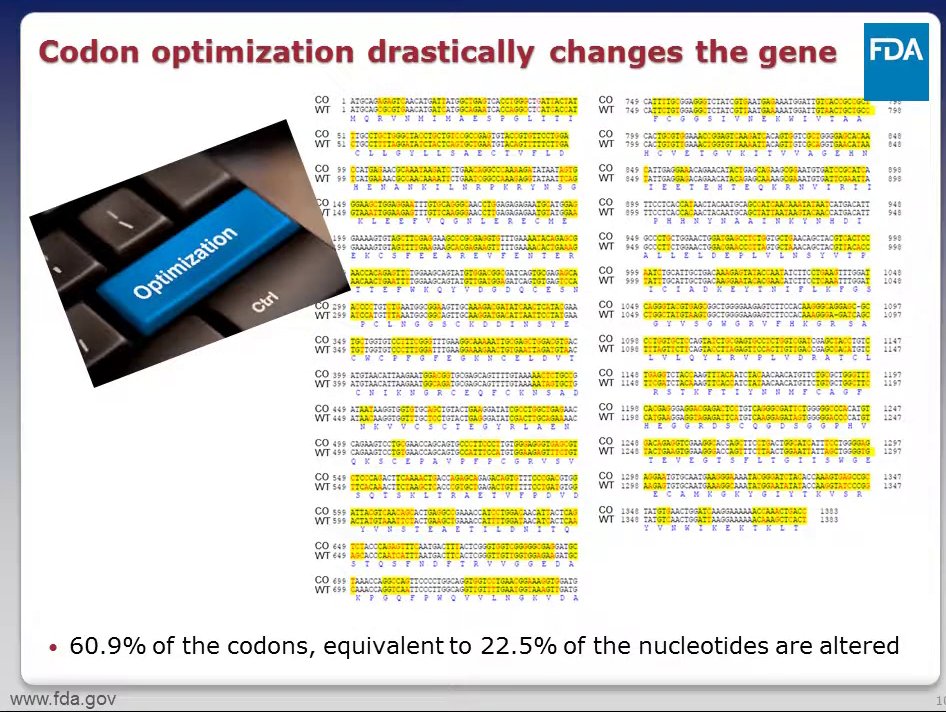
Same? Well, not so much.
In 2011 Nature Medicine magazine published an article called "Breaking the Silence". It described how codon optimization, which uses this synonymous DNA changes, can trigger disease in a number of ways.
doi.org/10.1038/nm1211…
#COptiGate
In 2011 Nature Medicine magazine published an article called "Breaking the Silence". It described how codon optimization, which uses this synonymous DNA changes, can trigger disease in a number of ways.
doi.org/10.1038/nm1211…
#COptiGate
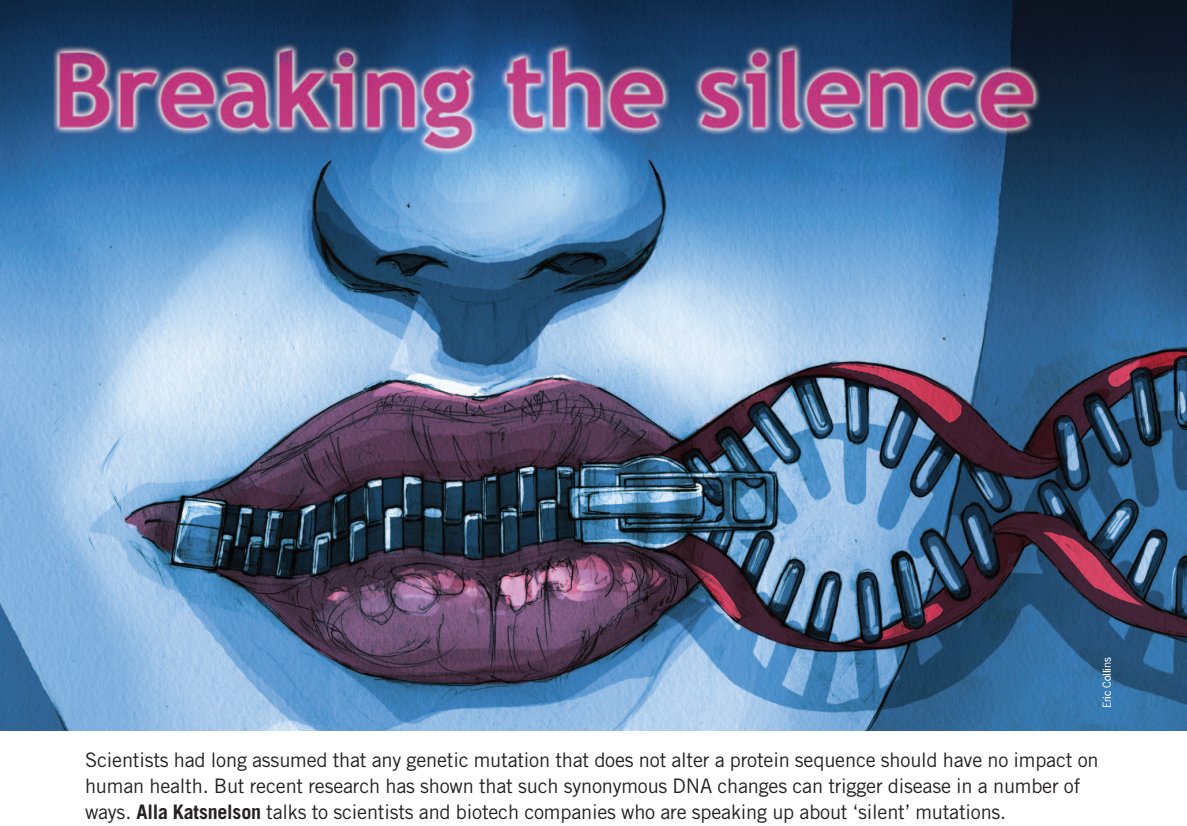
Turns out the protein which was manufactured when codon optimization has different ways it folds and a different 3D shape, and it "could cause immunogenicity, for example, which wouldn’t be seen until late-stage clinical trials or even after approval".
#OptiGate
#OptiGate

"The changed form could cause immunogenicity, for example, which wouldn’t be seen until late-stage clinical trials or even after approval." (Chava Kimchi Sarfaty, FDA)
This statement relates to the NORMAL approval cycle. The COVID vaccines went via an accelerated one.
#COptiGate
This statement relates to the NORMAL approval cycle. The COVID vaccines went via an accelerated one.
#COptiGate
"codon optimization can lead to alterations in protein conformation and function…. and increase immunogenicity….some of these elements can … alter protein folding, and lead to changes in protein conformation and post-translational modifications.” (Vincent P. Mauro)
#COptiGate
#COptiGate
Protein misfolding "has been linked with neurodegeneration in Alzheimer and Parkinson disease, and many other pathologies."
pubmed.ncbi.nlm.nih.gov/28441058/
#COptiGate
pubmed.ncbi.nlm.nih.gov/28441058/
#COptiGate
"The data confirm that protein misfolding resulting in intracellular PAO accumulation is sufficient to cause cardiomyocyte death and heart failure."
pubmed.ncbi.nlm.nih.gov/18612262/
#COptiGate
pubmed.ncbi.nlm.nih.gov/18612262/
#COptiGate
So if it is so problematic, why do manufacturers use it? because "higher levels of protein expression are required for clinical trials and commercialization, and these expression levels can sometimes be obtained by using (codon optimization)" (Vincent P. Mauro, 2018)
#COptiGate
#COptiGate
Pfizer is the most aggressive in their genetic code optimization (as far as we know); just read the abstract from "BNT162b2 Vaccine: Possible Codons Misreading, Errors in Protein Synthesis and Alternative Splicing's Anomalies"
doi.org/10.22541/au.16…
#COptiGate
doi.org/10.22541/au.16…
#COptiGate
Do they mention it to the regulator? no.
Here is Pfizer BNT162b2/Comirnaty Risk Management Plan for the EMA.
Variant V8 & V9 were tested, only difference was codon optimization, V8 had elevated levels of gamma-glutamyl transferase (GTT), V9 didn't.
#COptiGate


Here is Pfizer BNT162b2/Comirnaty Risk Management Plan for the EMA.
Variant V8 & V9 were tested, only difference was codon optimization, V8 had elevated levels of gamma-glutamyl transferase (GTT), V9 didn't.
#COptiGate


So Pfizer admits codon optimization can lead to elevated GTT, and "elevated GGT is linked to increased risk to a multitude of diseases and conditions, including cardiovascular disease, diabetes, metabolic syndrome (MetS), and all-cause mortality."
ncbi.nlm.nih.gov/pmc/articles/P…
ncbi.nlm.nih.gov/pmc/articles/P…
And even though Pfizer admits codon optimization impacts the safety of their product, "Safety pharmacology, genotoxicity and carcinogenicity studies have not been conducted in accordance with the 2005 WHO vaccine guideline."
How did they manage to avoid testing?
#COptiGate
How did they manage to avoid testing?
#COptiGate

The WHO 2005 document states that such tests normally not needed for the FINAL vaccine formulation. This is because in a NORMAL vaccine approval pharmacology, genotoxicity and carcinogenicity studies are done during ANIMAL STUDIES, which were practically skipped here.
#COptiGate
#COptiGate
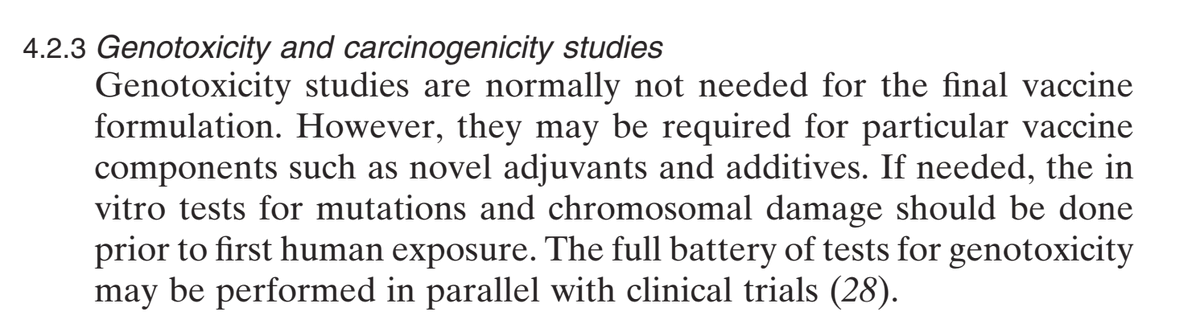
Even though EMA states:
"It is important to investigate the potential for undesirable pharmacological activity in appropriate
animal models and, where necessary, to incorporate particular monitoring for these activities in the
toxicity studies and/or clinical studies"
#COptiGate

"It is important to investigate the potential for undesirable pharmacological activity in appropriate
animal models and, where necessary, to incorporate particular monitoring for these activities in the
toxicity studies and/or clinical studies"
#COptiGate


Back to sequencing: this concern was reported in 2006, published in 2007, and "breaking the silence" was published in Nature Medicine magazine in 2011, the FDA or its equivalent in Europe (EMA) STILL THEY DO NOT HAVE a guidance with regard to the genetic sequencing.
#COptiGate
#COptiGate
Here is Katerina Alexaki from the FDA explaining how a SINGLE synonymous mutation (mutation that doesn't impact the protein but its 3D object & folding) can result in a disease and that if you have multiple substations there is a good chance it may have an effect.
#COptiGate
#COptiGate
Here is again Katerina Alexaki, this time answering the question whether the regulator demand the manufacturers to test for the impact of their codon optimization.
The answer is no.
#COptiGate
The answer is no.
#COptiGate
Here is a slide from a workshop given to the EMA in 2016, by FDA employee ("Immunogenicity of Biological Therapeutics Product Quality Attributes")
Construct design affects product quality and "Codon optimization and protein folding" are mentioned.
#COptiGate

Construct design affects product quality and "Codon optimization and protein folding" are mentioned.
#COptiGate

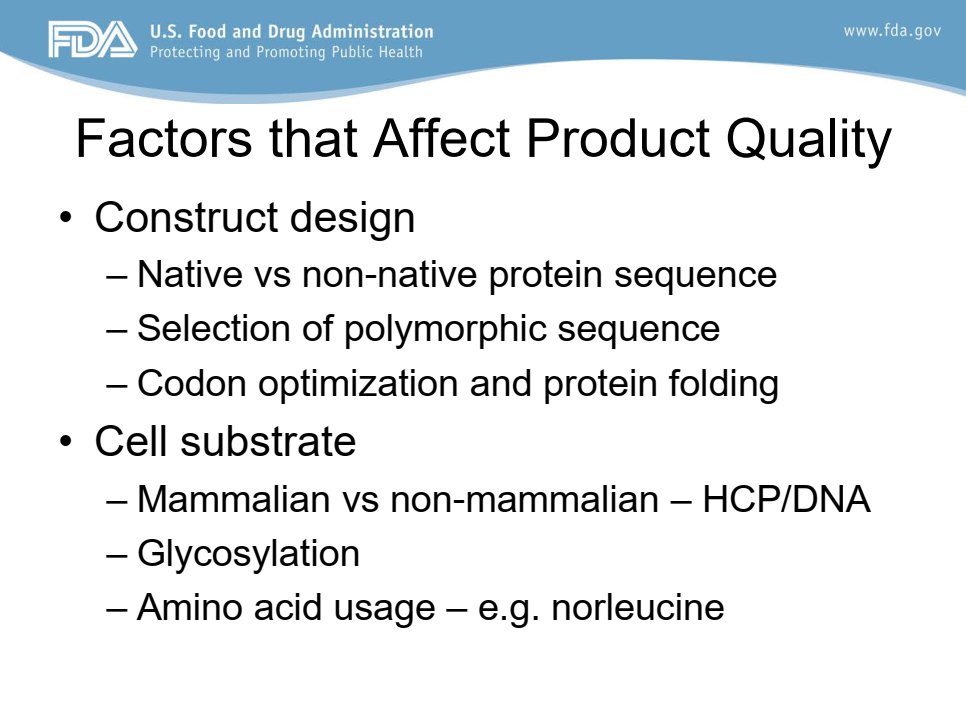
The manufacturers knows about the potential risk.
The regulators knows about the potential risk.
Yet regulators don't test V products as gene therapy, and do not put in place codon optimization risk mitigation plan.
IF YOU DON'T MEASURE RISK IT DOESN'T GO AWAY.
#COptiGate
The regulators knows about the potential risk.
Yet regulators don't test V products as gene therapy, and do not put in place codon optimization risk mitigation plan.
IF YOU DON'T MEASURE RISK IT DOESN'T GO AWAY.
#COptiGate

This topic was first discussed in my blog. You can find more information on the topic.
Please join my telegram channel t.me/eh_den
To those who contacted me and asked how they can support my work - thank you! I'll publish it soon!
senseofawareness.com/2021/08/09/var…
#COptiGate
Please join my telegram channel t.me/eh_den
To those who contacted me and asked how they can support my work - thank you! I'll publish it soon!
senseofawareness.com/2021/08/09/var…
#COptiGate
THE BONUS !!!
Could it be that the "variants" that we see are a result of the misfolding of the spike protein, which is a result of the codon optimization technology used?
Could it explain the correlation between vaccinations campaigns & "new variants" outbreaks?
#COptiGate
Could it be that the "variants" that we see are a result of the misfolding of the spike protein, which is a result of the codon optimization technology used?
Could it explain the correlation between vaccinations campaigns & "new variants" outbreaks?
#COptiGate
BONUS (cont)
If codon optimization is causing "new variants" (new symptoms & sickness), than any new product (eg boosters) which will include new codon optimized genetic code, will again lead to more forms of sickness.
PS
Where is the 3D model of the "Delta variant"?
#COptiGate
If codon optimization is causing "new variants" (new symptoms & sickness), than any new product (eg boosters) which will include new codon optimized genetic code, will again lead to more forms of sickness.
PS
Where is the 3D model of the "Delta variant"?
#COptiGate
Protein folding is governed by Gibbs Free Energy (ΔΔG). Protein stability seems to plays a vital role in the evolution of SARS-CoV-2. Dominant variants were found to exhibit significantly lower ΔΔG, with HIGHER THAN EXPECTED protein stability.
#COptiGate
biorxiv.org/content/10.110…
#COptiGate
biorxiv.org/content/10.110…
Just to explain the above, out of the variants of concern, NONE of the dominant mutations (67 of 19440 possible mutations) observed in induce a strongly destabilizing, which was significantly different to the expected 34% of possible mutations meeting this threshold.
#COptiGate
#COptiGate
(cont): "We suggest that protein folding calculations offer a useful tool for early identification of advantageous mutations"
Could it be that manufacturers used codon optimization and protein folding calculations in order to stabilize the selected spike protein?
#COptiGate
Could it be that manufacturers used codon optimization and protein folding calculations in order to stabilize the selected spike protein?
#COptiGate
"hold on, that's the spike protein, that does not explain the spread of the virus. The spike protein is not supposed to replicate!"
Agreed, but it does not explain IgG antibodies against the nucleocapsid in vaccinated non-infected people.
#COptiGate
academic.oup.com/cid/advance-ar…

Agreed, but it does not explain IgG antibodies against the nucleocapsid in vaccinated non-infected people.
#COptiGate
academic.oup.com/cid/advance-ar…
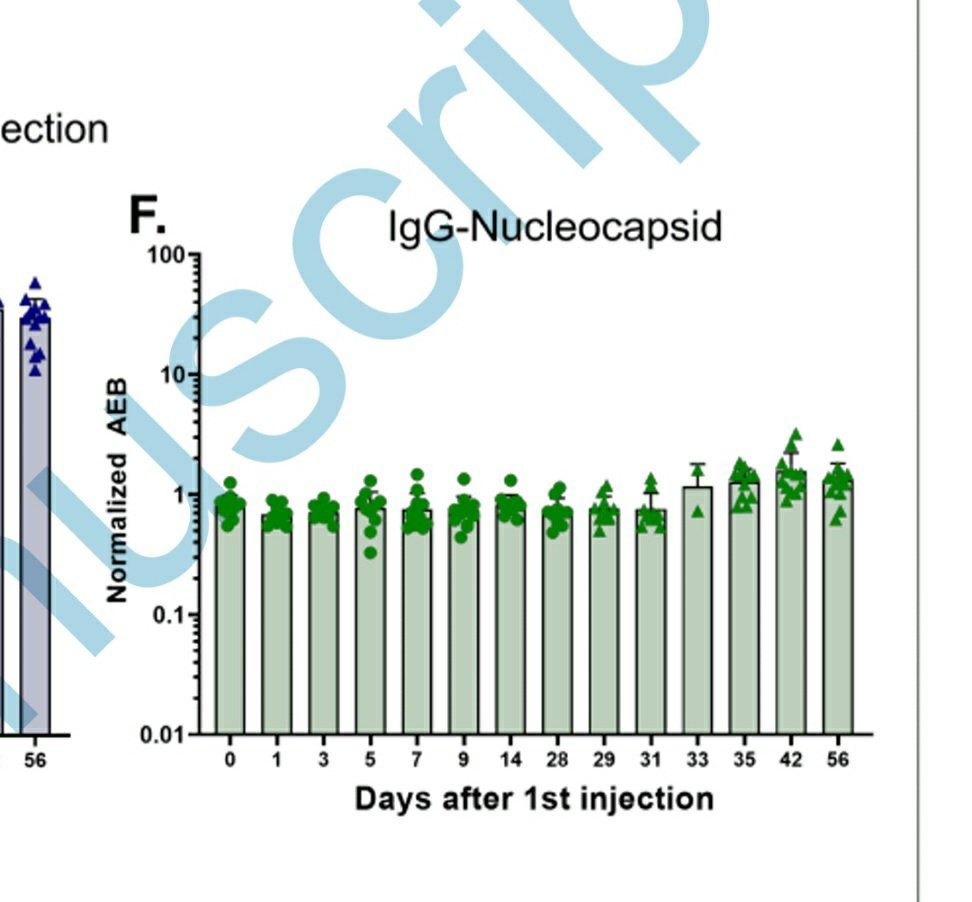
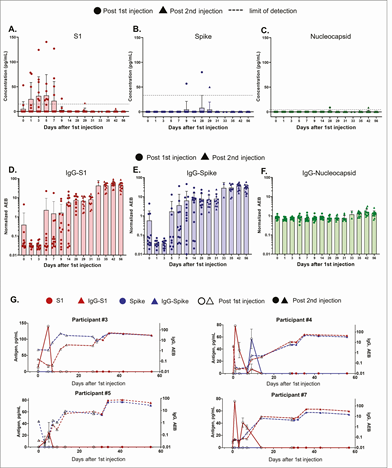
And for anyone who is still not freaked out...I forgot to mention reverse transcription, thank you @HiveAnti for reminding me.
"Breaking Study Sheds More Light on Whether an RNA Vaccine Can Permanently Alter DNA".
#OptiGate
sciencewithdrdoug.com/2021/02/15/bre…
"Breaking Study Sheds More Light on Whether an RNA Vaccine Can Permanently Alter DNA".
#OptiGate
sciencewithdrdoug.com/2021/02/15/bre…
COPTIGATE REDUX - MAJOR UPDATE!
The following tweets are A MAJOR REVISION / UPDATE to the story. It's darn complex.
As the doctor always says in the joke: "I've got some good news, and I've got some bad news."
Don't worry, you will get ANOTHER BONUS at the end !!!
#COptigate
The following tweets are A MAJOR REVISION / UPDATE to the story. It's darn complex.
As the doctor always says in the joke: "I've got some good news, and I've got some bad news."
Don't worry, you will get ANOTHER BONUS at the end !!!
#COptigate
Let's start with the GOOD news:
MOST pharmaceutical companies realized they have a problem (Actually since the MERS outbreak). The spike protein was unstable, so this time they inserted genetic code to create a supportive "skeleton" structure. (cont)
#COptiGate
MOST pharmaceutical companies realized they have a problem (Actually since the MERS outbreak). The spike protein was unstable, so this time they inserted genetic code to create a supportive "skeleton" structure. (cont)
#COptiGate
This is how it works:
They made two substitutions in the genetic code, adding two molecules of amino acid called proline, in order for it to act as an anchor to keep the protein structure in place.
I some MOST because AstraZeneca didn't...
Problems solved, right?
#COptiGate
They made two substitutions in the genetic code, adding two molecules of amino acid called proline, in order for it to act as an anchor to keep the protein structure in place.
I some MOST because AstraZeneca didn't...
Problems solved, right?
#COptiGate
Obviously not (BAD NEWS)!
So it seems that ALL the vaccines that are in the market who use this technology, known as S-2P or 2P, suffer from instability and it is difficult to produce reliably in mammalian cells. Just a reminder - we are (still) mammals.
#COptiGate
So it seems that ALL the vaccines that are in the market who use this technology, known as S-2P or 2P, suffer from instability and it is difficult to produce reliably in mammalian cells. Just a reminder - we are (still) mammals.
#COptiGate
Also, remember - we have TWO substitutions here. If ONE synonymous mutation can lead to a disease, what is the impact of TWO substitutions?
The new suggested structure, HexaPro, uses 6 substitutions.
Great for performance and stability, but what about safety?
#COptiGate
The new suggested structure, HexaPro, uses 6 substitutions.
Great for performance and stability, but what about safety?
#COptiGate

When you look at the actual reporting to EMA, you see that Moderna and Janssen (Ad26.COV2.S) reported the use of S-2P, AstraZeneca confirmed it doesn't... and Pfizer described the multiple modifications, and nobody raised it as a risk.
#COptiGate



#COptiGate




The regulator DOES NOT HAVE a guidance with regard to the genetic sequencing, does not have a process & toolset to measure the risks, and therefore, is unable to develop a Risk Management Program to address the probability & magnitude of future adverse events.
#COptiGate
#COptiGate
And again, as I said before, we have multiple substations that are right now being kept together by two proline molecules unstable and difficult to produce reliably, and in the case of AZ, not even held at all.
Does this makes you feel secure?
#COptiGate
science.sciencemag.org/content/369/65…
Does this makes you feel secure?
#COptiGate
science.sciencemag.org/content/369/65…
OHH.... ONE LAST THING....
Remember the report of Pfizer from Japan that showed how the vaccine lipids which contain the mRNA in them are leaving the injection site and spreading all around the body?
Guess what - it's worse than you think!
files.catbox.moe/0vwcmj.pdf
#COptiGate
Remember the report of Pfizer from Japan that showed how the vaccine lipids which contain the mRNA in them are leaving the injection site and spreading all around the body?
Guess what - it's worse than you think!
files.catbox.moe/0vwcmj.pdf
#COptiGate
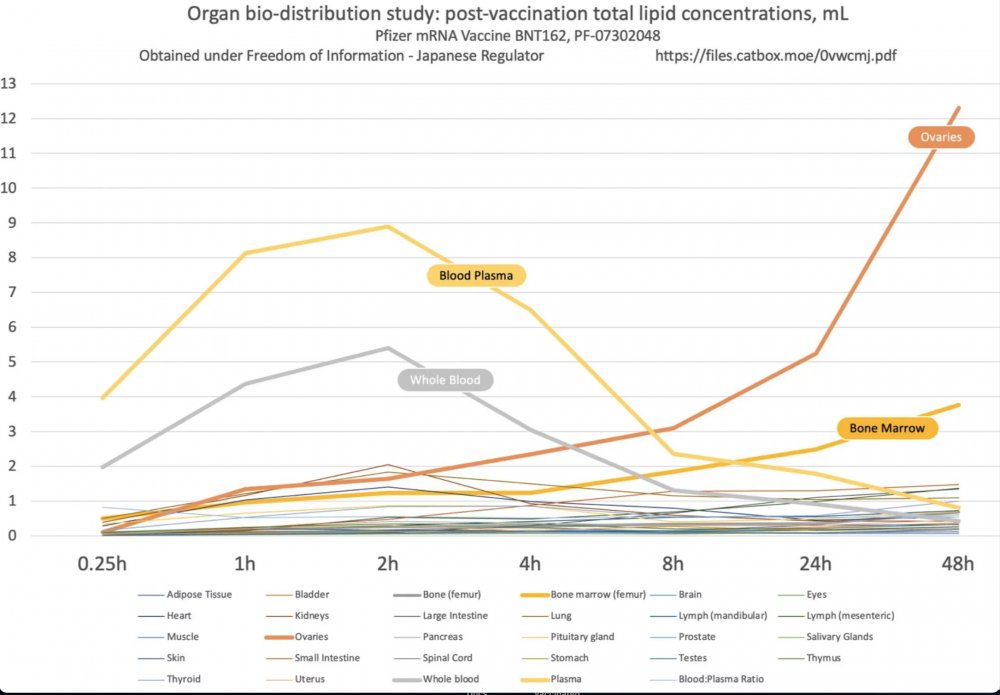
"Different cell types have drastically different coder usage, different tRNA levels, and figuring the translational kinetics in one tissue tells you NOTHING about the translational kinetics in a different tissue" (Katerina Alexaki)
#COptiGate
#COptiGate
Translational kinetics can lead to translational pauses, which have a role in modulating protein conformation, can lead to structural changes, increase immunogenicity and change performance. This has been reported since the 90’s.
Safe, right?
#COptigate
doi.org/10.1038/s41598…
Safe, right?
#COptigate
doi.org/10.1038/s41598…
There is a database of 51 types of tissues with known different coder usage. NO ONE tested their product vs all the different tissue types. NO ONE knows what the impact is.
THE REGULATOR MUST STOP ACTING LIKE A RUBBER STAMP.
THIS IS OUR BODIES, WE DEMAND SAFETY!
#COptiGate
THE REGULATOR MUST STOP ACTING LIKE A RUBBER STAMP.
THIS IS OUR BODIES, WE DEMAND SAFETY!
#COptiGate
WARNING - IF YOU ARE FROM ISRAEL, YOU ARRIVED TO THE WORST PART OF THIS THREAD !!!
REMEMBER: IGNORANCE IS BLISS: IF YOU CHOOSE TO CONTINUE READING, YOU WILL NEVER BE ABLE TO SEE REALITY AS BEFORE (I MEAN IT!!!)
#COptiGate
REMEMBER: IGNORANCE IS BLISS: IF YOU CHOOSE TO CONTINUE READING, YOU WILL NEVER BE ABLE TO SEE REALITY AS BEFORE (I MEAN IT!!!)
#COptiGate
On the 17th of December Pfizer informed they already supplied 2.9 million dosages, have millions more doses sitting in their warehouse, and claim they can deliver 50 million more by end of 2020.
#COptiGate
#COptiGate

Israel started its campaign on 19 December 2020, with prime minister Benjamin Netanyahu being the first person in the country to receive the vaccine. In less than two weeks, over 10% of Israelis received their first dose. Israel's population is 9,364,000, meaning ~1M.
#COptiGate
#COptiGate
On the 9th of December the EMA was hacked. On the January the information was shared online.
However, before we will go to them, let us get some context.
reuters.com/article/us-ema…
#COptiGate
However, before we will go to them, let us get some context.
reuters.com/article/us-ema…
#COptiGate
The European Medical Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) released a report on 19 February 2021 on the Pfizer product.
There is a section called "Steps taken for the assessment of the product".
#COptiGate


There is a section called "Steps taken for the assessment of the product".
#COptiGate



Remember this report was written 3 MONTHS after Israel already started its vaccination program. In Israel, on the 19th of February 4,280,046 got 1 shot, 2,932,180 got 2nd shot, in total 7,212,226 shots.
datadashboard.health.gov.il/COVID-19/gener…
#COptiGate
datadashboard.health.gov.il/COVID-19/gener…
#COptiGate

Back to EMA report:
Prizer had two processes: process 1 (clinical trial material) and Process 2 (commercial process).
The two processes generated different products:
"It can therefore not be concluded that identical species are obtained by the processes."
#COptiGate
Prizer had two processes: process 1 (clinical trial material) and Process 2 (commercial process).
The two processes generated different products:
"It can therefore not be concluded that identical species are obtained by the processes."
#COptiGate

The product included fragmented mRNA, which expected not to cause problems:
"It is likely that the fragmented species will not result in expressed proteins, due to their expected poor stability and poor translational efficiency"
COptiGate
"It is likely that the fragmented species will not result in expressed proteins, due to their expected poor stability and poor translational efficiency"
COptiGate

Fragments are supposed to lack segments that will allow it to be fully processed, but it's not the case.
"majority of fragments...expected to be ...truncated
transcripts...the results indicating a substantial proportion ... are not in agreement with this statement."
#COptiGate
"majority of fragments...expected to be ...truncated
transcripts...the results indicating a substantial proportion ... are not in agreement with this statement."
#COptiGate

In summary: the product does work in the cell as it is supposed to.
"most fragments would arise from premature termination in the IVT (In vitro Transcription) reaction."
#COptiGate
"most fragments would arise from premature termination in the IVT (In vitro Transcription) reaction."
#COptiGate

poly(A) acts as a timer of mRNA stability, and its value was not tested for during production.
"The length of the tails is important for RNA stability and translational efficiency the poly(A) tail length should be included to the active substance release testing"
#COptiGate
"The length of the tails is important for RNA stability and translational efficiency the poly(A) tail length should be included to the active substance release testing"
#COptiGate

Remember the hack?
One of the emails has identified “a significant difference in % RNA integrity/truncated species” between the clinical batches and proposed commercial batches—from around 78% to 55%."
#COptiGate
doi.org/10.1136/bmj.n6…
One of the emails has identified “a significant difference in % RNA integrity/truncated species” between the clinical batches and proposed commercial batches—from around 78% to 55%."
#COptiGate
doi.org/10.1136/bmj.n6…
MUCH, MUCH WORSE:
"THE POSSIBILITY OF TRANSLATED PROTEINS OTHER THAN THE INTENDED SPIKE PROTEIN (S1S2), RESULTING FROM TRUCATED AND/OR MODIFIED mRNA SPECIES SHOULD BE ADDRESSED"
In other words: THE POSSIBILITY OF MUTATIONS MUST BE ADDRESSED.
#COptiGate
"THE POSSIBILITY OF TRANSLATED PROTEINS OTHER THAN THE INTENDED SPIKE PROTEIN (S1S2), RESULTING FROM TRUCATED AND/OR MODIFIED mRNA SPECIES SHOULD BE ADDRESSED"
In other words: THE POSSIBILITY OF MUTATIONS MUST BE ADDRESSED.
#COptiGate
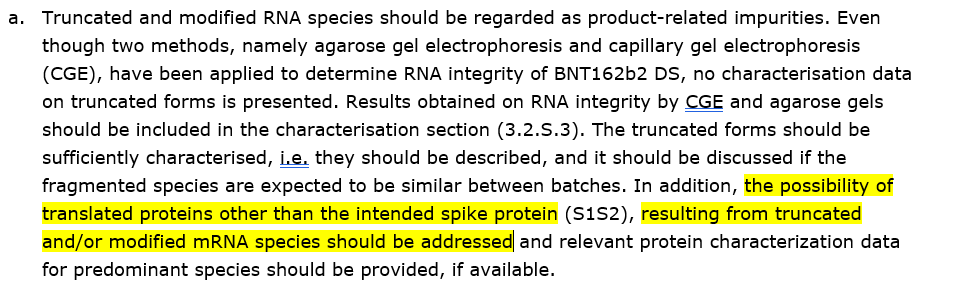
Remember Israel? 7,212,226 shots were given to 4,280,046 individuals of a product that suffered from the above manufacturing problems.
PFIZER KNEW ABOUT IT. DO YOU THINK ISRAEL DIDN'T?
Do you still wonder why the product is not serialized?
#COptiGate
PFIZER KNEW ABOUT IT. DO YOU THINK ISRAEL DIDN'T?
Do you still wonder why the product is not serialized?
#COptiGate
Reading the leaked document I quoted from (BWP Rolling Review report to ETF / CHMP, Rolling Review #2) is actually good risk measurement, it covers a lot of areas, but it does not cover Codon Optimization at all.
#COptiGate
covidvaccinereactions.com/ema-pfizer-lea…
#COptiGate
covidvaccinereactions.com/ema-pfizer-lea…
Manufacturers are having SO MUCH PROBLEMS to deliver a product that will be in line with cGMP requirements (which is everything above), so I understand why they want to completely ignore a huge threat landscape.
But why is the regulator not demanding to check it?
#COptiGate
But why is the regulator not demanding to check it?
#COptiGate
There are people (at least in the FDA) such as Katerina Alexaki who understand the topic and have developed a good process of validating the performance of Codon Optimization in biotherapeutics.
So WHY the regulator (FDA/EMA) don't test it?
#COptiGate
So WHY the regulator (FDA/EMA) don't test it?
#COptiGate
From my professional experience, refusal to measure risk IS ALWAYS an indication that someone knows there is a real problem. ALWAYS.
It ties back to #PfizerLeak: no liability for big pharma + full liability to country = no incentives to talk about the new elephant.
#COptiGate
It ties back to #PfizerLeak: no liability for big pharma + full liability to country = no incentives to talk about the new elephant.
#COptiGate
I believe that the real reason why people are not acting professionally is political pressure, social pressure, and a spinning door between regulators & industry.
The systems we have in place suppress honest people. This is how disasters occur.
THIS NEEDS TO STOP.
#COptiGate
The systems we have in place suppress honest people. This is how disasters occur.
THIS NEEDS TO STOP.
#COptiGate
I ALMOST MISSED THE PROOF!!!!
EMA was asking for:
"RELEVANT PROTEIN/PEPTIDE CHARACTERIZATION DATA FOR PREDOMINANT SPECIES SHOULD BE PROVIDED, IF AVAILABLE"
THE REGULATOR DON'T KNOW (or at least didn't know) WHAT THE mRNA IS CREATING, not to mention codon information.
#COptiGate

EMA was asking for:
"RELEVANT PROTEIN/PEPTIDE CHARACTERIZATION DATA FOR PREDOMINANT SPECIES SHOULD BE PROVIDED, IF AVAILABLE"
THE REGULATOR DON'T KNOW (or at least didn't know) WHAT THE mRNA IS CREATING, not to mention codon information.
#COptiGate


Beloved fellow human beings!
I invite you to read this thread.
Love,
Ehden
I invite you to read this thread.
Love,
Ehden
https://twitter.com/eh_den/status/1427235192385806342
Hi,
If you reached so far, well done!
I welcome you to read the follow up to the story - more on codon optimization, why the chances that the SARS_CoV_2 is a "product of nature" are WAY LESS than 1 in 844,596,301... and much more.
#COptiGate
If you reached so far, well done!
I welcome you to read the follow up to the story - more on codon optimization, why the chances that the SARS_CoV_2 is a "product of nature" are WAY LESS than 1 in 844,596,301... and much more.
#COptiGate
https://twitter.com/eh_den/status/1428102982839721992
Hi again,
After reading my COPTIGATE - 3D CHESS EDITION post, please visit my thread about the Pfizer mRNA code analysis, and if you can, consider supporting my work:
PayPal: paypal.me/ehdenbiber
Bitcoin: bit.co.in/ehden
patreon.com/ehden
After reading my COPTIGATE - 3D CHESS EDITION post, please visit my thread about the Pfizer mRNA code analysis, and if you can, consider supporting my work:
PayPal: paypal.me/ehdenbiber
Bitcoin: bit.co.in/ehden
patreon.com/ehden
https://twitter.com/eh_den/status/1431674294606651393
• • •
Missing some Tweet in this thread? You can try to
force a refresh













