Early IFN-α signatures and persistent dysfunction are distinguishing features of NK cells in severe COVID-19, bit.ly/3teIlId, our latest team effort in #COVID19 research lead by Jacob Natterman @LabSchultze and @AschenbrennerAC out now @ImmunityCP. [1/n]
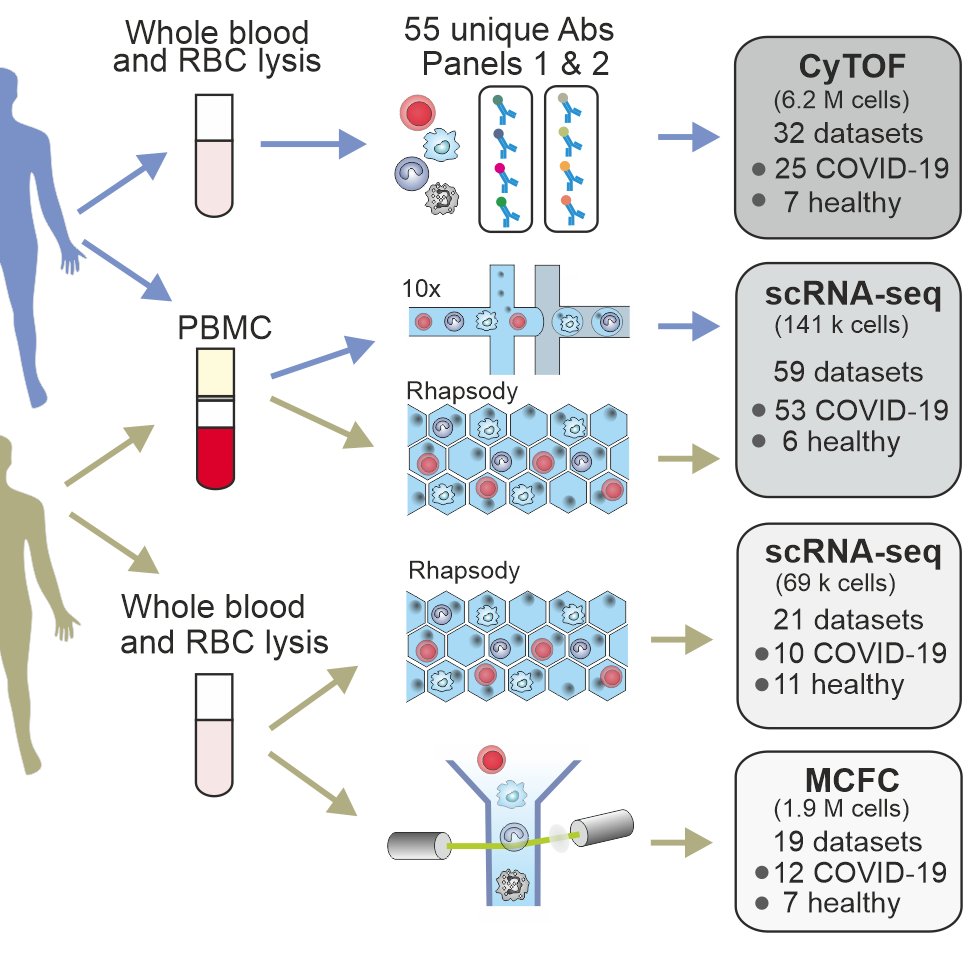
We performed a detailed characterization of natural killer cells in 205 patients from four independent cohorts using #scRNAseq, #MCFC and #CyTOF together with functional studies. [2/n]
We found elevated IFN-α plasma levels in early severe COVD-19 alongside increased NK cell expression of ISGs and genes involved in IFN-α signaling. [3/n]
NK cells exert anti-SARS-CoV-2 activity but are functionally impaired in severe COVID-19. [4/n]
Further, NK cell dysfunction may be relevant for development of fibrotic lung disease in severe COVID-19, as NK cells exhibit impaired anti-fibrotic activity. [5/n]
Our study associates prolonged IFN-α-induced NK cell response with poorer disease outcome. [6/n]
Once more an intense and fruitful collaboration with the groups of @Sander_Lab and Birgit Sawitzki within the Deutsche COVID-19 OMICs Initiative decoi.eu… [7/n]
… Big shouts to all first authors who made it possible: @BenniKraemer, @knoll_rainer, @LorenzoBonaguro, Jan Raabe, Michael ToVinh… [8/n]
• • •
Missing some Tweet in this thread? You can try to
force a refresh