This thread is about our new preprint on transposable element called long interspersed nuclear elements (LINE-1/L1). When expressed excessively in the cerebellum, L1 causes ataxia (impaired coordination).
A fascinating finding by @taka_takehiro 👇🏽 (1/)
biorxiv.org/content/10.110…
A fascinating finding by @taka_takehiro 👇🏽 (1/)
biorxiv.org/content/10.110…
Human genome is occupied in large part by transposable elements or jumping genes. LINE-1 occupies ~20% of our genome, compared to only 1.1% by exons. Some evolutionarily young LINE-1 are still active and are a rare cause of genetic diseases. (2/)
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…
In addition, L1 may even promote the process of aging & age-related diseases in humans. Until recently, research focused on their activity in the germline. Now, their activity in somatic tissues during a lifespan is being studied. Fascinating review👇🏽(3/)
nature.com/articles/s4158…
nature.com/articles/s4158…
How do we “live” with these transposable element? The human cells repress L1 by epigenetic silencing mediated by molecules like the HUSH complex, TRIM28 and DNMT1. However, repression is not perfect. (4/)
pubmed.ncbi.nlm.nih.gov/29728366/
pubmed.ncbi.nlm.nih.gov/29728366/
@taka_takehiro became interested in L1 in adult tissues. A recent study of the developing human brain demonstrated that cerebellum retains exceptionally high expression of key epigenetic silencers of TEs such as TRIM28 and DNMT1. (5/)
genome.cshlp.org/content/31/9/1…
genome.cshlp.org/content/31/9/1…
Cerebellum is a major structure of the hindbrain located near the brainstem important in motor control. Damage to the cerebellum (genetic or otherwise) can cause ataxia - impaired coordination.(6/)
mayoclinic.org/diseases-condi…
mayoclinic.org/diseases-condi…

A rare genetic mutation called ataxia telangiectasia (AT) is caused by mutations in the ATM gene important for DNA repair. Important studies showed that ATM regulates the retrotransposition activities of LINE-1. (7/)
pnas.org/content/108/51… nature.com/articles/cr201…
pnas.org/content/108/51… nature.com/articles/cr201…
@taka_takehiro examined autopsy cerebellum tissues of AT patients and compared with other forms of ataxia. He found that AT patients’ cerebella expressed higher levels of L1 with reduced expression of TRIM28 and DNMT1. A striking inverse correlation btw L1 - TRIM28 was seen. (8/) 



To determine whether L1 over expression alone can cause ataxia, @taka_takehiro went onto generate L1 over expression mouse. He used CRISPRa mouse model to induce L1 expression. There is a remarkable 4 year story behind this strategy but save that for another day. (9/) 
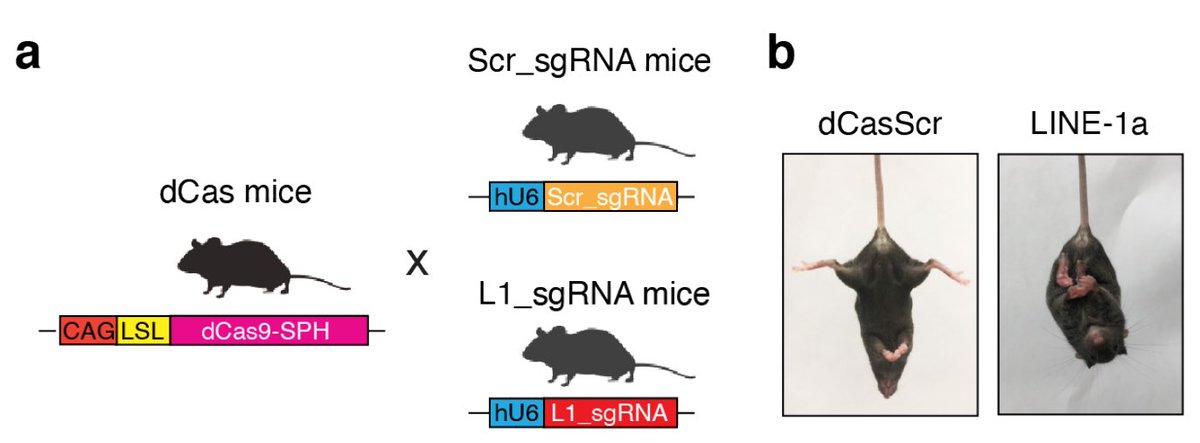
Having generated L1 activation mouse, he examine where the L1 Orf1 protein is expressed in the cerebellum. Orf1a is mainly expressed in the Purkinje neurons (absolutely beautiful cells 🤩) in the cerebellum. (10/) 
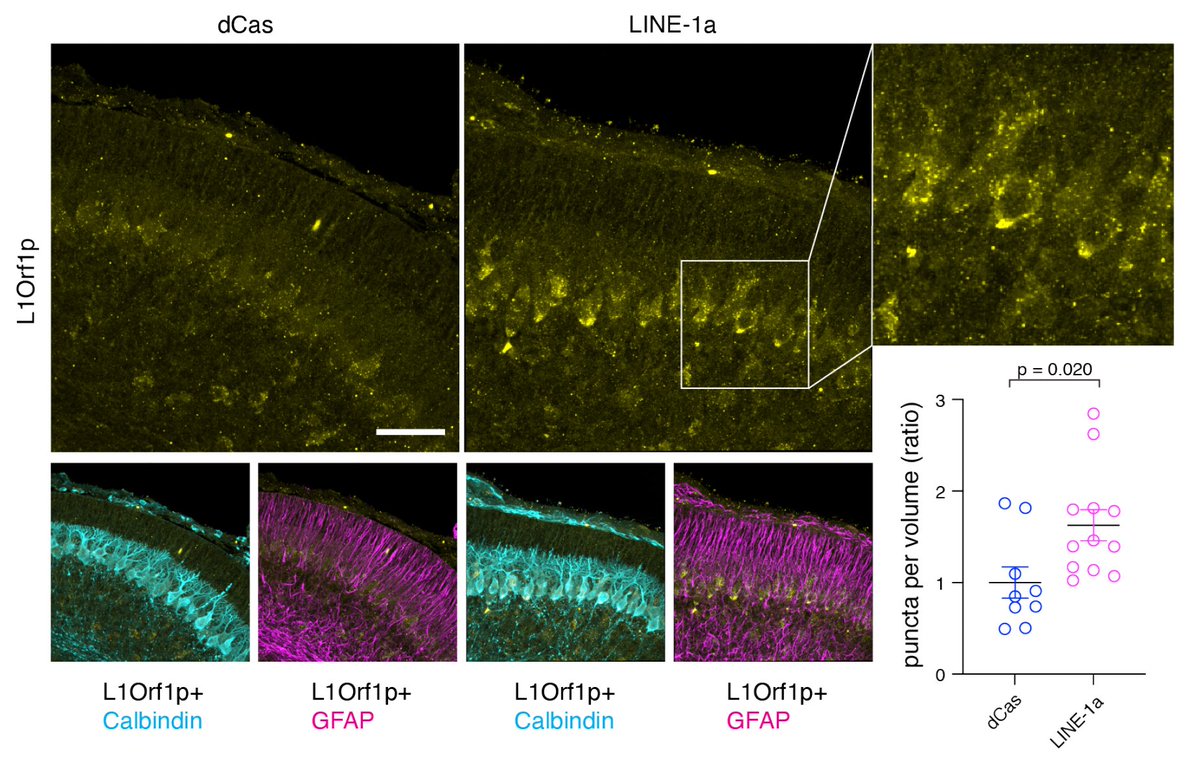
What are their phenotypes? The LINE-1 active mice develop rapid progressive ataxia within 4-8 weeks of birth. The most robust forms of a genetic mouse model of ataxia we have seen. (11/) 

What happens to the neurons with LINE-1 activated? The Purkinje cells of LINE-1a mice exhibit robust functional impairment accompanied by DNA damage, ER stress, and eventual loss. Brilliant work of collaborators in the Tamas Horvath lab 💪🏼(12/) 



Can we do anything to treat these mice? We tested reverse transcriptase inhibitor, 3TC, which blocks cDNA synthesis from L1 mRNA. 3TC treatment starting at 4 week of age was able to prevent ataxia progression and preserve motor coordination in these L1 active mice! (13/) 

Awesome work by @taka_takehiro Eriko Kudo @ericsongg @fernandocarvphd Yuki Yasumoto Yong Kong @annsea_park Milan Stoiljkovic Xiao-Bing Gao Marya Shanabrough Klara Szigeti-Buck Zhong-Wu Liu Yalan Zhang Parker Sulkowski Peter Glazer Leonard Kaczmarek & Tamas Horvath 👏🏼 👏🏼 (14/)
This study illustrates the danger of LINE-1 dysregulation in the brain, and highlights that neurological diseases can have their origins in transposons and viral infections. Based on our work, antiretroviral agents may be beneficial in preventing ataxia progression in AT. (End)
• • •
Missing some Tweet in this thread? You can try to
force a refresh