1/Truly inspiring and [by far] my favorite talk (so far) @Midwinter_Immun
quick 🧵
quick 🧵
https://twitter.com/Midwinter_Immun/status/1485668708659785731
2/Kagan and co. address the outstanding question: How can you activate and program a dendritic cell to promote protective (including antitumor) immunity?
Same as, why have DC-based vaccines and all cancer vaccines failed thus far?
Same as, why have DC-based vaccines and all cancer vaccines failed thus far?
3/The key is in keeping the DC alive and active during stimulation.
Most adjuvants (e.g. LPS, alum, polyI:C) induce pyroptosis which (while triggering local inflammation) limits the activity and costimulatory potential of a dendritic cell
Most adjuvants (e.g. LPS, alum, polyI:C) induce pyroptosis which (while triggering local inflammation) limits the activity and costimulatory potential of a dendritic cell
4/According to Kagan, TLR stimulation is insufficient,
You need to also activate the NLRP3 ➡️ IL-1 pathway as well as promote migration to the draining lymph nodes
To orchestrate effective T cell priming
You need to also activate the NLRP3 ➡️ IL-1 pathway as well as promote migration to the draining lymph nodes
To orchestrate effective T cell priming
5/One mode of stimulation fulfilling this checklist of signals are oxidized phospholipids oxPAPC (data presented included PGPC
More info in this review ➡️ nature.com/articles/s4157…

More info in this review ➡️ nature.com/articles/s4157…


6/ PGPC (and other ox-phospholipids) generate hyperactive DCs; a term used to describe that DCs that are both active and alive
compared to pyroptotic ones which are inactive (e.g. in the case of Alum or LPS-alone)
compared to pyroptotic ones which are inactive (e.g. in the case of Alum or LPS-alone)
7/These hyperactive DCs (LPS + PGPC) promote markedly superior antitumor immunity compared to other adjuvants
(unfortunately, this really striking data remains unpublished)
(unfortunately, this really striking data remains unpublished)
8/The hyperactive DC must express both inflammasomes and CCR7 for this programming and subsequent immunity to occur a
These DCs are strikingly better at stimulating CD8 T cell responses (showed a 3-fold increase in Ag-specific CD8 responses, especially at higher Ag doses)
These DCs are strikingly better at stimulating CD8 T cell responses (showed a 3-fold increase in Ag-specific CD8 responses, especially at higher Ag doses)
9/As a side-note, J.K. presented the interesting finding that there is a different requirement for CD8 and CD4 T cells for anti-tumor immunity between young and old mice
CD8 ➡️ young
CD4 ➡️ old
CD8 ➡️ young
CD4 ➡️ old
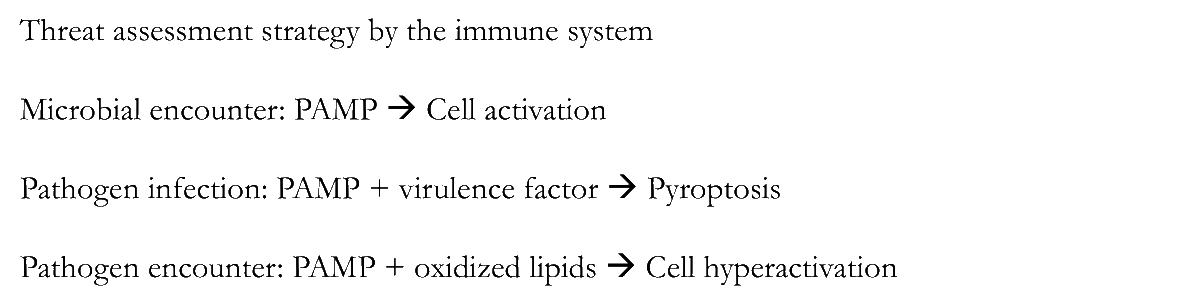
10/Coming back to the main point, Kagan integrated this data well into the PAMP model
(➡️ bit.ly/Janeway2002)
Juxtaposed to infection (➡️bit.ly/VanceIsbergPor…)
(➡️ bit.ly/Janeway2002)
Juxtaposed to infection (➡️bit.ly/VanceIsbergPor…)
12/While the model strongly supports and builds upon the Janeway concepts and data, "threat" is reminiscent of Matzinger's Danger theory
science.org/doi/10.1126/sc…

science.org/doi/10.1126/sc…


13/Two questions of importance that were raised:
One: Do Macrophages exhibit the same "hyperactive" state when challenged with oxPL?
Answer: No, DCs are unique to this response.
Macrophages are prone to pyroptosis either way
Ralph Steinman must be smiling, somewhere.
One: Do Macrophages exhibit the same "hyperactive" state when challenged with oxPL?
Answer: No, DCs are unique to this response.
Macrophages are prone to pyroptosis either way
Ralph Steinman must be smiling, somewhere.
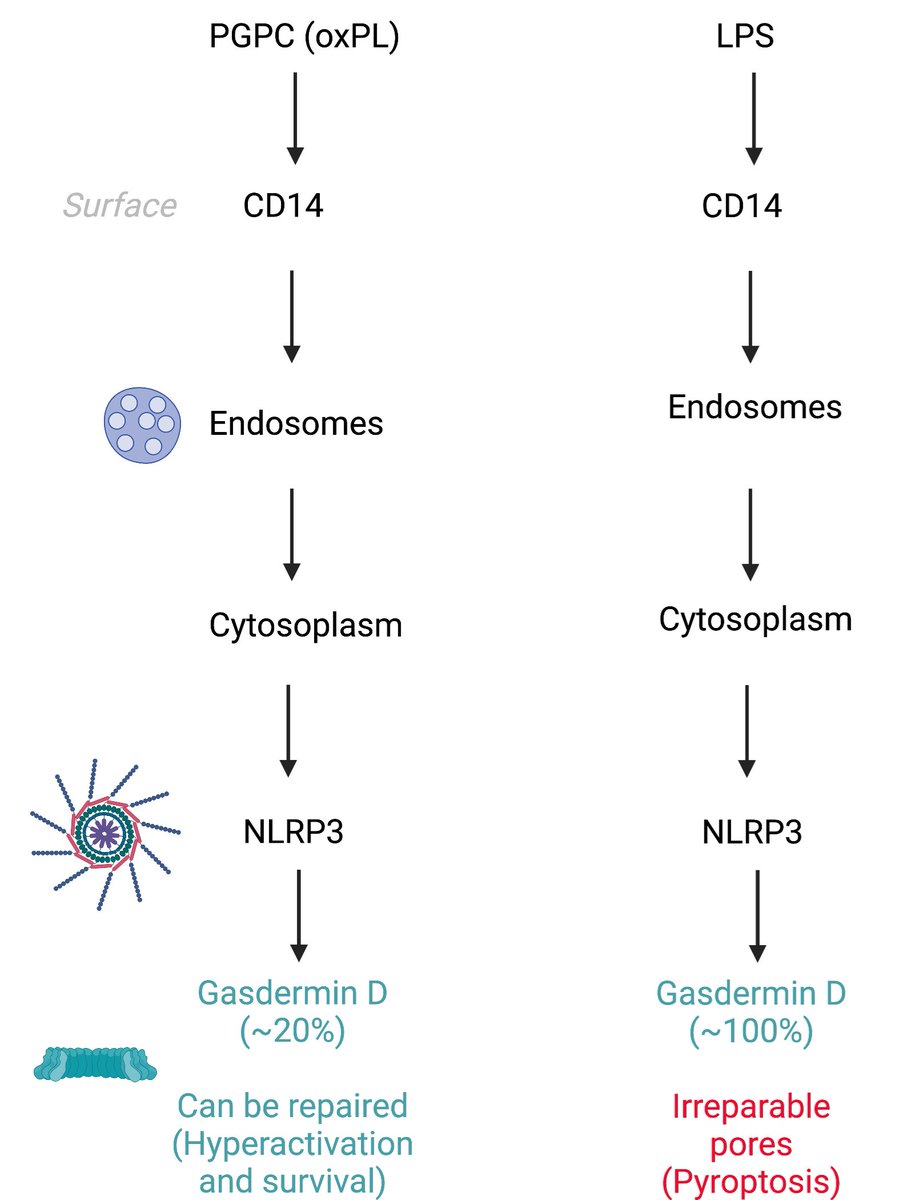
14/ Two: What are the signaling mechanisms that distinguish a "pyroptotic" vs "hyperactive" response?
Answer: It's about how much Gasdermin D gets activated. A diagram I quickly made of what I understood to be the answer
Answer: It's about how much Gasdermin D gets activated. A diagram I quickly made of what I understood to be the answer
15/One point I wanted to ask (and think) about is whether we have control over whether lipids get "oxidized" vs "peroxidized" in vivo.
One would think lipid peroxidation could trigger ferroptosis whereas oxidized lipids would hyperactivate.
Just my uneducated guess.
One would think lipid peroxidation could trigger ferroptosis whereas oxidized lipids would hyperactivate.
Just my uneducated guess.
16/As I mentioned in earlier tweets, the signals impacting the DC fate to potentiate their ability to drive a certain T cell response is probably one of the most important remaining questions in lymphocyte biology, with implications ranging from vaccines to tolerance.
• • •
Missing some Tweet in this thread? You can try to
force a refresh