
For those wondering how the newest kid on the block, LY-CoV1404 (bebtelovimab) neutralizes variants so well, including #SARSCoV2 #Omicron BA.1.1 and BA.2? The answer is – pure luck! This antibody is like Neo dodging bullets in the matrix…🍀🍀🍀 

The binding mode is very similar to imdevimab (REGN10987) but bebtelovimab avoids major imdevimab resistance caused by the N440K Omicron mutation! It also avoids other mutations. Think social distancing of antibodies from mutations... 
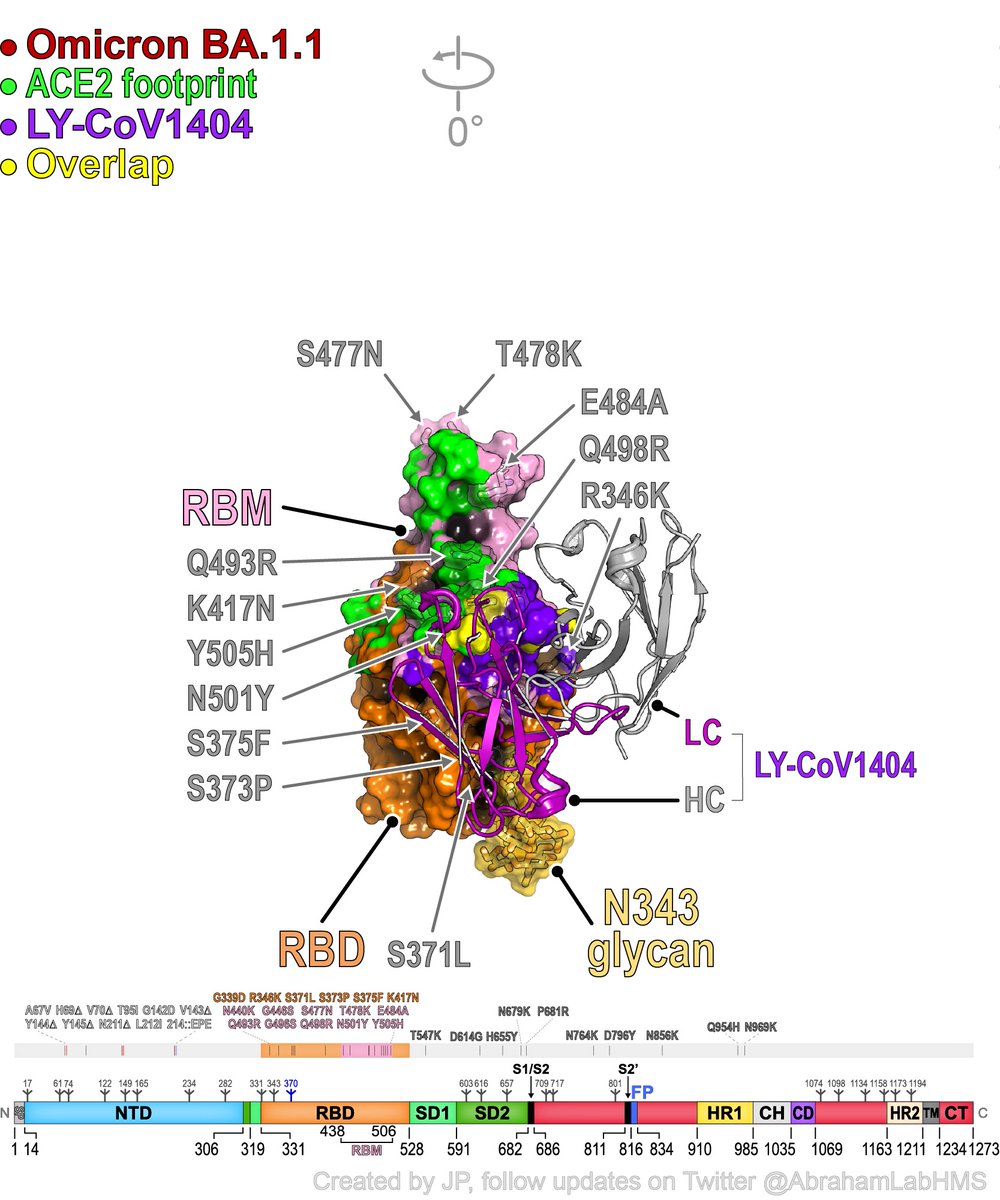
Can use this 📺 to scroll through and see #Omicron BA.1.1 #SARSCoV2 mutations and where #antibodies bind... Sotrovimab/S309, Casirivimab/REGN10933, Imdevimab/REGN10987, Evusheld (AZD8895/tixa, AZD1061/cilga), Bam (LY-CoV555), Ete (LY-CoV016), and bebtelovimab (LY-CoV1404)
For those wanting to learn more, here's the actual preprint for the structure - beautiful work *not* done by our lab, we just like to visualize...and predict what kind of #SARSCoV2 #evolution might come next...😱 PDB: 7MMO. biorxiv.org/content/10.110…
• • •
Missing some Tweet in this thread? You can try to
force a refresh



