$PVCT Releases 2023 Stockholder Letter globenewswire.com/news-release/2… #rosebengal #rosebengalsodium
1/ $PVCT 2023 Shareholder Letter: (2) Design, prepare, and potentially commence a Phase 2/3 RCT of PV-10®+SOC checkpoint vs monotherapy SOC checkpoint ($MRK #keytruda, $BMY #opdivo) for 1st-line Stage III cutaneous melanoma. #rosebengal #rosebengalsodium. A THREAD.
2/ Utilizing clinical data from an ongoing, multi-cohort, Phase 1b/2 study of PV-10+checkpoint ($MRK #keytruda) for checkpoint-naïve metastatic melanoma (NCT02557321). #rosebengal #rosebengalsodium.
3/ Cohort #1, main cohort (MC): PV-10+#keytruda for checkpoint-naïve Stage IV melanoma. provectusbio.com/media/docs/pub…. 2 Stage IIIC-D patients were also treated. #rosebengal #rosebengalsodium.
4/ Cohort #2, first expansion cohort (EC1): PV-10+#keytruda for checkpoint-refractory in advanced melanoma. provectusbio.com/media/docs/pub…. Late-Stage III and Stage IV patients. #rosebengal #rosebengalsodium.
5/ Cohort #3, second expansion cohort (EC2): PV-10+#keytruda for checkpoint-naive Stage III melanoma (including patients with in-transit melanoma). provectusbio.com/media/docs/Mel…. 2 patients in MC + 4 patients in EC2. #rosebengal #rosebengalsodium.
6/ Data from PV-10+#keytruda for checkpoint-naïve Stage III melanoma: globenewswire.com/news-release/2…. #rosebengal #rosebengalsodium.
7/ Stage III melanoma -- Patient-level 50% CR and 83% ORR by RECIST 1.1 criteria in 6 subjects; CRs rapidly developed within 15 to 27 weeks (within ~6 months). #rosebengal #rosebengalsodium.
8/ Stage III melanoma -- Durable CRs with a median PFS that was not reached during the 2-year treatment interval, an 83% PFS rate, and all CRs ongoing after 18 to 36 months of study follow-up. #rosebengal #rosebengalsodium.
9/ Stage III melanoma -- Patient CRs prognostic of survival, a median OS that was not reached, and a 100% OS rate for CRs that were ongoing after 18 to 36 months of study follow-up. #rosebengal #rosebengalsodium.
10/ $PVCT believes one active immunotherapy drug agent (PV-10) was paired with another ($MRK #keytruda #pembrolizumab) in 3 distinct patient cohorts in NCT02557321.
11/ NCT02557321 patient cohorts were enrolled ~sequentially, as part of the historical study design: MC starting in 2015, EC1 in 2018, and EC2 in 2019. This $PVCT’s first clinical trial combining PV-10 with a checkpoint inhibitor. #rosebengal #rosebengalsodium.
12/ For historical context, a group of stockholders (PRH) entered into a Definitive Financing with $PVCT in 2017. prnewswire.com/news-releases/…. NCT02557321’s original 2014/15 protocol contemplated only 5 PV-10 cycles per patient & reinjection of baseline tumor burden until lesion CR.
13/ Protocol lessons-learned from all NCT02557321 cohorts included giving PV-10 as needed (pro re nata/PRN) after an initial treatment course & injecting/re-injecting new lesions above baseline tumor burden until lesion CR, which could lead to patient CR in most-to-all patients.
14/ There are 3 primary classes of checkpoints: CTLA-4 (#yervoy) first approved in 2011. 2 PD-1s (#keytruda, #opdivo) first approved in 2014. A number of other PD-(L)1s that are approved or investigational, and a LAG-3 agent approved in combination with #opdivo (#opdualag).
15/ $PVCT had to show clinical safety of a PV-10 checkpoint combination (#keytruda). AEs were consistent with the established patterns for the single-agent use of each drug: ~Grade 1-2 injection site reactions for PV-10 and ~Grade 1-3 immune-mediated reactions for #keytruda.
16/ All NCT02557321 cohorts showed that, as a combination therapy, $PVCT’s PV-10’s safety (AE) profile was consistent and “orthogonal” to (ie, non-overlapping with) $MRK’s #keytruda. #rosebengal #rosebengalsodium.
17/ $PVCT’s PV-10’s clinical activity/efficacy in combination with a checkpoint for checkpoint-naïve Stage IV melanoma had comparable EP data to other combination therapies involving a local/systemic cancer agent and a checkpoint. ESMO 2019 poster presentation image below. 
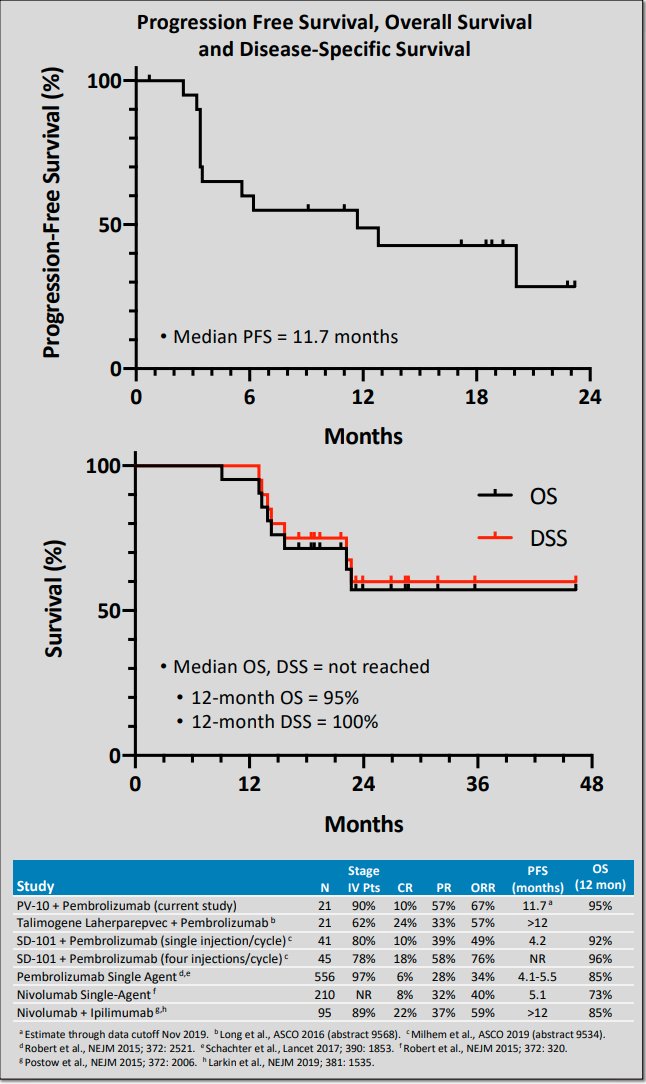
18/ Several melanoma combination therapies vs monotherapy checkpoint have failed. See Daud at SITC 2022, Melanoma Failed CPI Trials (IDO, TVEC, BEMPEG, Idera, Combi I, IMSpire). sitcancer.org/2022/program/a….
25/ What makes $PVCT believe that intratumoral PV-10+checkpoint in melanoma can beat monotherapy checkpoint?
26/ $PVCT 1. Is PV-10 active? 2. What is PV-10’s MOA? 3. What is PV-10’s immune MOA? 4. Is PV-10 an immunotherapy? 5. What is PV-10’s checkpoint combination therapy MOA? 6. Is PV-10 synergistic with a checkpoint? #rosebengal #rosebengalsodium.
27/ (/26-1) $PVCT “The preliminary clinical data provided in your request for [BTD] are indicative of drug activity in the treatment of local, satellite or in-transit recurrence of malignant melanoma…” (2014). sec.gov/Archives/edgar….
28/ 2014 was not the “best way” to find out that $PVCT’s PV-10 has drug activity.
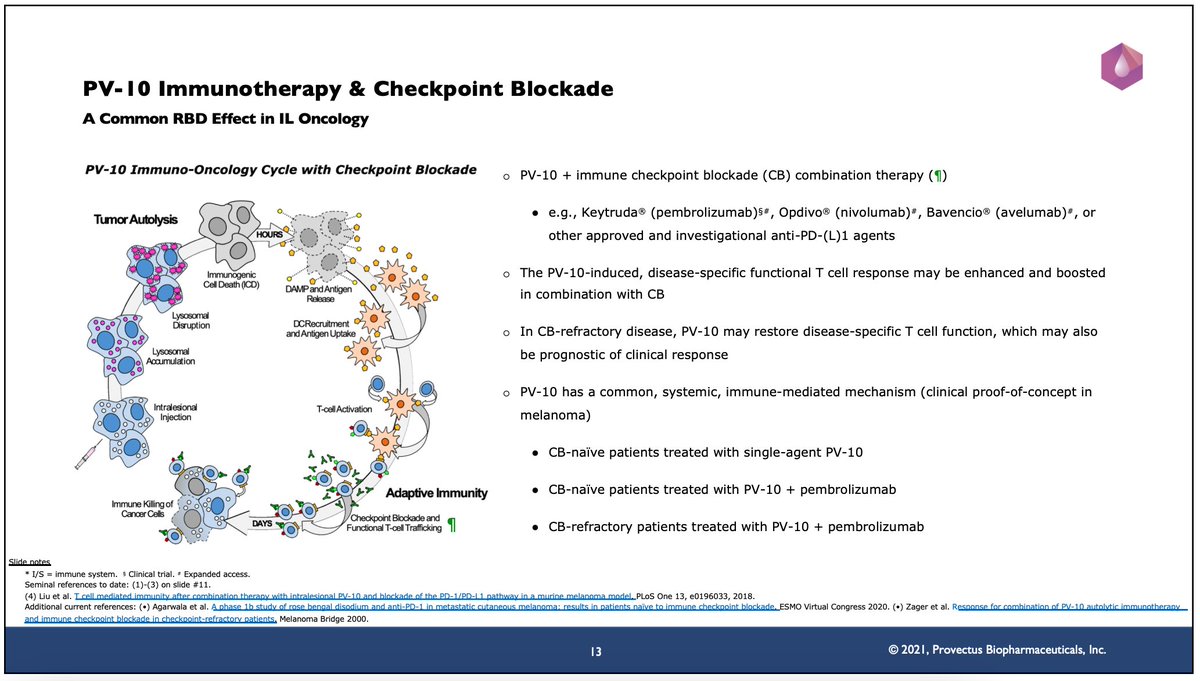
29/ (/26-2) $PVCT intelligent cytotoxicity: PV-10 injection in cancer tumors initiates
tumor autolysis within HOURS of tumor injection. The rapid accumulation of PV-10 in tumor lysosomes triggers lysosomal disruption, and then immunogenic cell death (ICD).
tumor autolysis within HOURS of tumor injection. The rapid accumulation of PV-10 in tumor lysosomes triggers lysosomal disruption, and then immunogenic cell death (ICD).
30/ In a clinical setting, the key to PV-10’s success is injecting all of a patient’s tumors (all of a patient’s tumor heterogeneity) and achieving CR of those PV-10-injected tumors. The more tumors injected with PV-10 and the greater the lesion CR rate, the better.
31/ #yervoy anti-CTLA-4, #keytruda, #opdivo, #libtayo (anti-PD-1), #bavencio, #tecentriq, #imfinzi (anti-PD-L1), #yervoy+opdivo (CTLA-4+PD-1), #opduolag (PD-1+LAG-3) are all non-specific (non-tumor-specific) immunotherapies.
32/ Yarchoan NEJM (2017) begins to show this non-specificity/non-tumor specificity: nejm.org/doi/10.1056/NE…. Why does a checkpoint, a cancer immunotherapy, not work for all cancers?
33/ $PVCT believes that PV-10 enables “a person's own immune system to fight cancer,” but that PV-10 itself does not “…boost or change how the immune system works…” to “find and attack cancer cells.” cancer.org/treatment/trea….
34/ $PVCT believes that PV-10 is a tumor-specific cancer immunotherapy and that PV-10’s cytotoxicity of cancer cells (upon PV-10 injection into cancer tumors) conveys information to the immune system from the cells of the tumors. #rosebengal #rosebengalsodium
35/ (/24-3) $PVCT innate immune signaling: PV-10-induced ICD causes the release of DAMPs, cytokines, and tumor antigens, leading to DC recruitment and antigen uptake; PV-10-induced ICD also yields STING activation. #rosebengal #rosebengalsodium
39/ Returning to /26-2 PV-10 intelligent cytotoxicity. Multivariate cytotoxicity. #rosebengal #rosebengalsodium
40/ Panzarini (2014): #rosebengal “…induced multiple cell death pathways…apoptosis was the first preferred mechanism of death, and it was triggered by at least four different pathways...”
40/ Panzarini (2014): “…whose independent temporal activation ensures cell killing when one or several of the pathways are inactivated.” ncbi.nlm.nih.gov/pmc/articles/P…. #multiple. #rosebengal #rosebengalsodium.
41/ Swift (2019): “…PV-10 disrupts lysosomes…and induces apoptosis in a concentration-, time-, and cell-line-dependent manner…” dovepress.com/potent-in-vitr…. #reproducibility.
42/ Swift “In human adult cancer cell lines, PV-10 induces cell death by different mechanisms in a cell line-dependent manner. PV-10 induced cell death by primary necrosis in SW480 colorectal adenocarcinoma cells, by a combination of apoptosis and necrosis in...”
42/ Swift: “...HCT-116 colorectal carcinoma and HT-29 colorectal adenocarcinoma cell lines, and in three human primary melanoma cell samples and by primarily apoptosis in UWB ovarian carcinoma cells and AGS gastric adenocarcinoma cells.” #rosebengal #rosebengalsodium
43/ (/24-3) $PVCT adaptive immune response: Antigen presentation educates and activates T cells, leading to maturation into functional immune system assets: primarily CD8 cytotoxic T cells; also CD4 T cells, NKT cells & NK cells within the FIRST WEEK after PV-10 tumor injection.
44/ (23-4) Thus, $PVCT believes that PV-10 is an immunotherapy candidate – a tumor-specific cancer immunotherapy drug product candidate. #precisionmedicine. #rosebengal #rosebengalsodium.
45/ (/24-5) $PVCT’s PV-10’s checkpoint combination therapy’s MOA is “induce and boost.” The tumor-specific, functional T cell response induced by PV-10 injection of cancer tumors may be enhanced and boosted in combination with a checkpoint.
47/ Schmidt. Developing combination strategies using PD-1 checkpoint inhibitors to treat cancer (2018). link.springer.com/article/10.100….
48/ Independent contributions to combined action: “Synergistic effect.” By probability, the combined effect of independently active agents should be: Yab,P > Ya + Yb - (Ya × Yb). Z-score = Yab,P - (Ya + Yb – [Ya × Yb]).
49/ The higher the Z-score, the greater the contribution of the combination therapy. $PVCT calculated a Z-score of 25% for PV-10+#keytruda in 1st-line (ie, checkpoint-naive) Stage IV melanoma for the MC of NCT02557321.
1/ Potential synergy between $PVCT’s PV-10 and a checkpoint ($MRK’s #keytruda #pembrolizumab). A SUB-THREAD. #rosebengal #rosebengalsodium
2/ In Tweet 56 of THE MAIN THREAD, $PVCT notes that PV-10 has shown synergy in combination with a checkpoint for checkpoint-naïve Stage III and checkpoint-naïve Stage IV melanoma. See Tweets 47-50. #rosebengal #rosebengalsodium
3/ There can be 2 ways of looking at synergy. Casually: ORR of combination therapy Drug A + SOC Drug B > ORR of monotherapy SOC Drug B = synergy between Drugs A and B.
4/ Mathematically (like Schmidt 2019), ORR_AB > ORR_A + ORR_B - (ORR_A × ORR_B) {the Z-score} = synergy. This approach makes the casual view more about additivity than synergy. ncbi.nlm.nih.gov/pmc/articles/P….
5/ As noted in Tweet 49, the higher the Z-score, the greater the contribution of the combination therapy. $PVCT calculated a Z-score of 25% for PV-10+#keytruda in checkpoint-naïve Stage IV melanoma in NCT02557321. #rosebengal #rosebengalsodium
6/ What is the Z-score of PV-10+#keytruda in checkpoint-naïve Stage III melanoma in NCT02557321? $PVCT #rosebengal #rosebengalsodium
7/ A = $PVCT’s PV-10. B = $MRK’s #keytruda. ORR_AB = 83% (2022). ORR_A = 71% (2014), see provectusbio.com/media/docs/pub….
8/ What is ORR_B ($MRK’s #keytruda) for checkpoint-naïve Stage III melanoma?
9/ Nan Tie (2020) 58%? jitc.bmj.com/content/8/1/e0…. Z-score = -5%.
10/ AIM website reference: 24%? aimatmelanoma.org/stages-of-mela…. Z-score = +5%.
11/ Somewhere in between? $PVCT’s PV-10 and $MRK’s #keytruda’s synergy = -5% to 5%. There are of course lots of caveats (and assumptions) to this range of values.
12/ On the one hand, at the higher end of this range (ie, 5%), PV-10 may show synergy with #keytruda for checkpoint-naïve Stage III melanoma. $PVCT #rosebengal #rosebengalsodium
13/ In Tweet 10 of THE MAIN THREAD, $PVCT wrote that one active immunotherapy drug agent (PV-10) was paired with another ($MRK #keytruda #pembrolizumab) in NCT02557321. #rosebengal #rosebengalsodium
14/ Thus, on the other hand, at the lower end of this range (ie, -5%), one could say that (a) PV-10 is independently additive to #keytruda (since #pembrolizumab is SOC) or #keytruda is independently additive to PV-10 (since ORR_PV-10 > ORR_Keytruda). #rosebengal #rosebengalsodium
• • •
Missing some Tweet in this thread? You can try to
force a refresh