Statins, given like Halloween candy to those with >10% risk of CVD, can cause insulin resistance & diabetes.
A prime example of "double think".
This 🧵 explores how statins impede insulin sensitivity & damage our pancreas.
#statins #diabetes #T2DM #CVD #cholesterol
A prime example of "double think".
This 🧵 explores how statins impede insulin sensitivity & damage our pancreas.
#statins #diabetes #T2DM #CVD #cholesterol

Let's start off by reminding ourselves that diabetes and the broader category of metabolic syndrome is one of the leading risk factors for CVD.
Any drug that claims to be protective of CVD, should not be impeding insulin sensitivity.
Any drug that claims to be protective of CVD, should not be impeding insulin sensitivity.
Statins are associated with increased incidence of T2DM, 10-28% in some studies.
One way this happens is by a reduction in insulin secretion - a hormone that facilitates glucose uptake from the bloodstream into cells.
One way this happens is by a reduction in insulin secretion - a hormone that facilitates glucose uptake from the bloodstream into cells.

Animal models show that statins reduced insulin secretion alongside:
• Increased ROS production in pancreatic cells
• Disrupted membrane potential
• Mitochondrial swelling.
• Increased ROS production in pancreatic cells
• Disrupted membrane potential
• Mitochondrial swelling.
Unfortunately, pancreatic β-cells-cells have weak antioxidant systems relative to their oxidative burden, making them vulnerable to ROS accumulation.
This may be the self-same reason why myopathy is the most common side-effect of statins.
Muscle fibres are prone to ROS accumulation due to their preference for anaerobic respiration.
Muscle fibres are prone to ROS accumulation due to their preference for anaerobic respiration.
As I highlighted in my previous thread, statins can further impede antioxidant defences by CoQ10 and glutathione depletion.
https://twitter.com/sam_soete/status/1657013095510966272?s=20
In fact, statin-induced pancreatic dysfunction can be diminished with CoQ10 and L-Carnitine supplementation.
• CoQ10 regenerates antioxidants such as vitamin C & vitamin E.
• L-Carnitine is also an Antioxidant.
• CoQ10 regenerates antioxidants such as vitamin C & vitamin E.
• L-Carnitine is also an Antioxidant.
Lets dive even deeper into the metabolic pathways involved in insulin secretion, and how statins may be nuking them:
ATP synthesis is crucial for β-cells-cells because they regulate KATP channels.
ATP synthesis is crucial for β-cells-cells because they regulate KATP channels.
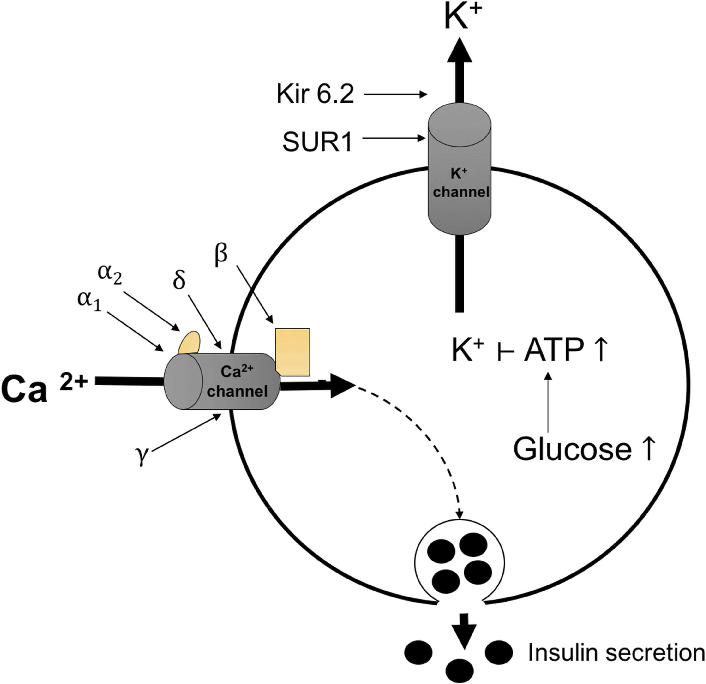
KATP channels (or ATP-sensitive potassium channels) are ion channels found in the cell membrane of various tissues in the body, including the pancreas, heart, and skeletal muscle (the same tissues arise again and again).
KATP channel closure initiates cell depolarization, regulating cellular excitability and subsequent insulin secretion.
Now that we know how important ATP synthesis is for β-cells, how might statins be impeding this production?
Statins have a time and dose-dependent mitochondrial dysfunction in the form of ETC complex impairment and reduced ATP synthase expression.
The time-dependent factor is crucial because many patients may only develop T2DM years after taking statins.
The time-dependent factor is crucial because many patients may only develop T2DM years after taking statins.

Isoprenoid led prenylation of ATP synthase is crucial to proper functioning of the enzyme.
Statins deplete isoprenoid biosynthesis such as farnesyl pyrophosphate and geranylgeranyl pyrophosphate due to impairment of the mevalonate pathway.
Statins deplete isoprenoid biosynthesis such as farnesyl pyrophosphate and geranylgeranyl pyrophosphate due to impairment of the mevalonate pathway.
Interestingly, mevalonate and NAC (antioxidant) can rescue insulin secretion in statin-induced pancreatic dysfunction.
Those that looked carefully at the KATP channel diagram a few tweets ago will have noticed the calcium channel on the left.
Statins may impair L-type voltage-gated calcium channels which prevents the cytosolic calcium spike that is crucial for insulin secretion.
Statins may impair L-type voltage-gated calcium channels which prevents the cytosolic calcium spike that is crucial for insulin secretion.
Simvastatin inhibited glucose-induced calcium signalling in rat pancreatic islet β-cells via direct blockage of L-type calcium channels, but this was not seen with pravastatin.
This suggests that effects are related to lipophilicity.
This suggests that effects are related to lipophilicity.
↑Blood glucose → ↑ATP levels → closure KATP channels → depolarization of the cell membrane → opening of voltage-gated calcium channels.
So statins act on both sides of the KATP channels to prevent insulin secretion.
So statins act on both sides of the KATP channels to prevent insulin secretion.
We have covered how statins reduce insulin secretion in depth, but lets take a look at how they impede the actions of insulin as well.
Insulin promotes glucose uptake by binding to insulin receptors on the surface of cells.
This binding activates a signalling cascade that culminates in the translocation of glucose transporter type 4 (GLUT4) to the cell membrane.
This binding activates a signalling cascade that culminates in the translocation of glucose transporter type 4 (GLUT4) to the cell membrane.

Statins reduce GLUT4 expression in adipocytes which can be salvaged to some extent by CoQ10 supplementation.
In skeletal muscle, glucose uptake is also facilitated by the activation of a protein called Akt (protein kinase B). Akt promotes the translocation of GLUT4 to the cell membrane.
Statins cause a reduced phosphorylation of Akt thereby disrupting the Akt pathway.
Statins cause a reduced phosphorylation of Akt thereby disrupting the Akt pathway.
How might these proposed mechanisms described above translate to T2DM risk?
• JUPITER trial: 20mg rosuvastatin for patients with >1 risk factor of DM ↑ 28% risk of T2DM
• CARDS study: 10mg atorvastatin mild ↑ in hyperglycaemia progression
• JUPITER trial: 20mg rosuvastatin for patients with >1 risk factor of DM ↑ 28% risk of T2DM
• CARDS study: 10mg atorvastatin mild ↑ in hyperglycaemia progression
Meta-analysis found a dose and time dependent association between statin use and T2DM.
Order of highest risk:
Rosuvastatin>atorvastatin>pravastatin
Order of highest risk:
Rosuvastatin>atorvastatin>pravastatin
TLDR:
• T2DM is a major risk factor for CVD
• Statins impede insulin secretion
• Statins impair insulin action
• Statins can cause T2DM
• T2DM is a major risk factor for CVD
• Statins impede insulin secretion
• Statins impair insulin action
• Statins can cause T2DM
If you are interested in statins then study these accounts carefully, the value provided is priceless:
• @holmanm
• @LDLSkeptic
• @DrAseemMalhotra
• @holmanm
• @LDLSkeptic
• @DrAseemMalhotra
If you learned something in this thread then:
1) Retweet the first tweet so others can benefit
2) Follow me to learn more about mitochondria and optimising health
1) Retweet the first tweet so others can benefit
2) Follow me to learn more about mitochondria and optimising health
https://twitter.com/sam_soete/status/1657880137155883011?s=20
• • •
Missing some Tweet in this thread? You can try to
force a refresh

 Read on Twitter
Read on Twitter




