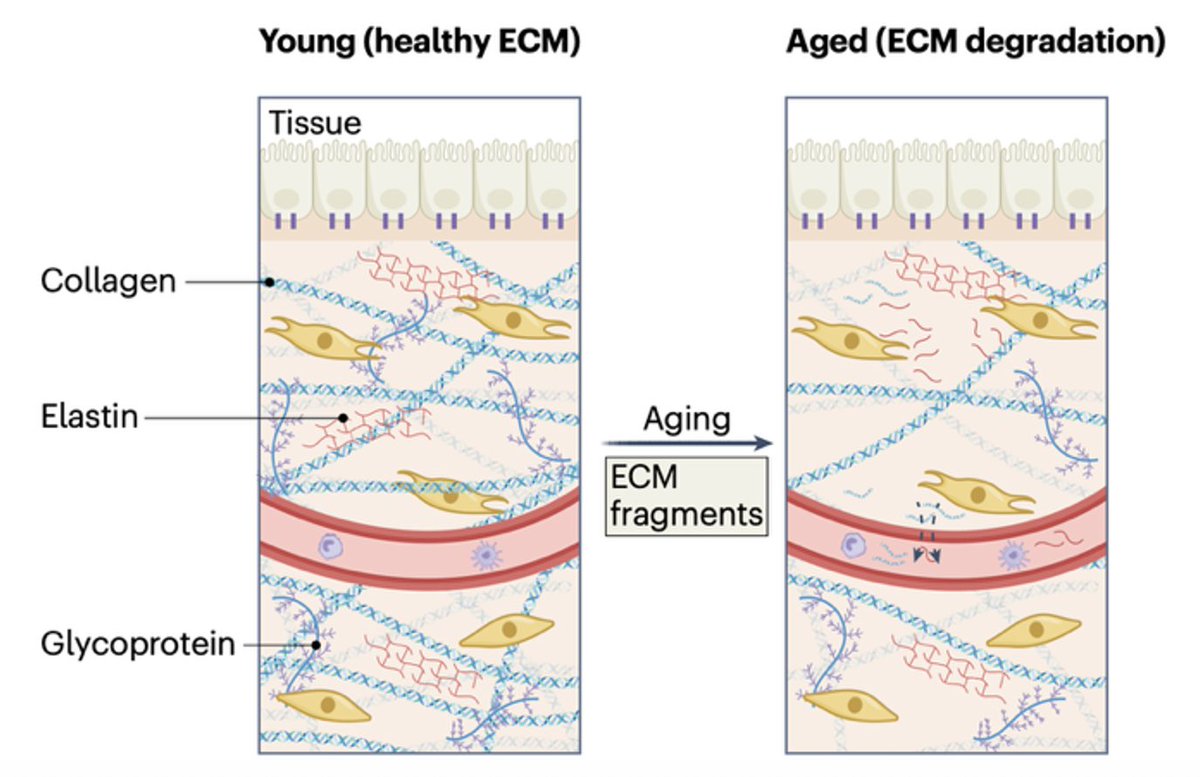
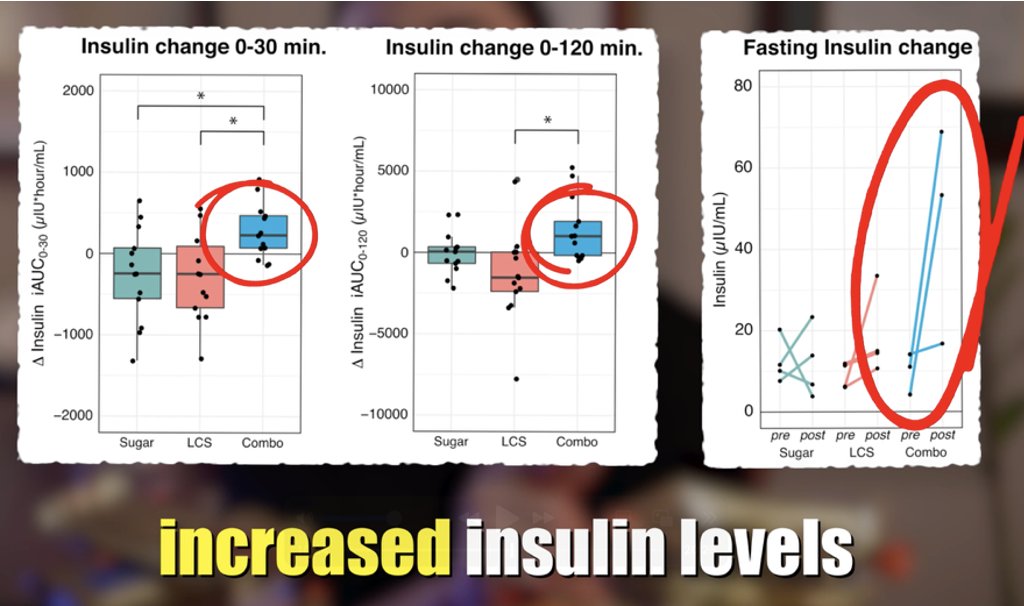
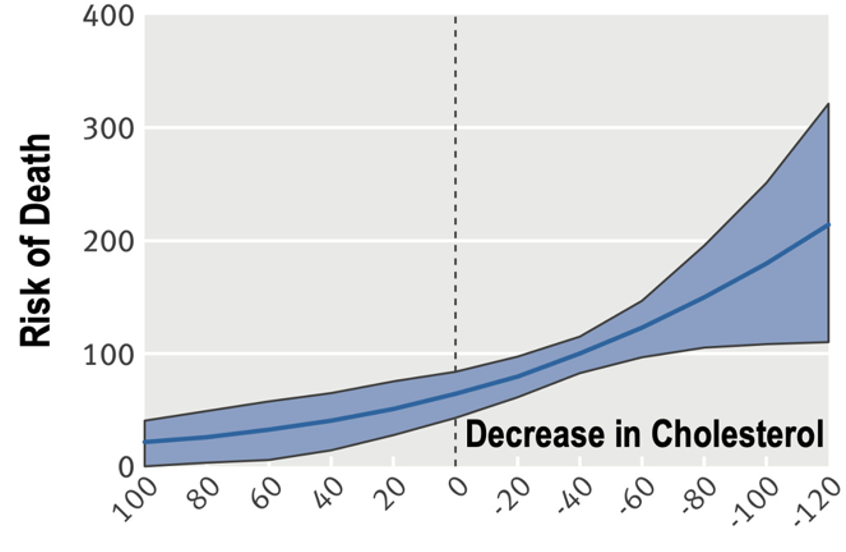
1/10) 🚨NEW! #Keto for Anorexia🚨
We report on 3 patients who achieved remission from treatment-resistant anorexia using animal-based keto diet 🥩🍳
👉BMIs 10 - 13 kg/m2
👉Each gained ≥20kg
👉+Dramatic improvements in mental health
insulinresistance.org/index.php/jir/…
Read & Share🧵🙏 twitter.com/i/web/status/1…
We report on 3 patients who achieved remission from treatment-resistant anorexia using animal-based keto diet 🥩🍳
👉BMIs 10 - 13 kg/m2
👉Each gained ≥20kg
👉+Dramatic improvements in mental health
insulinresistance.org/index.php/jir/…
Read & Share🧵🙏 twitter.com/i/web/status/1…
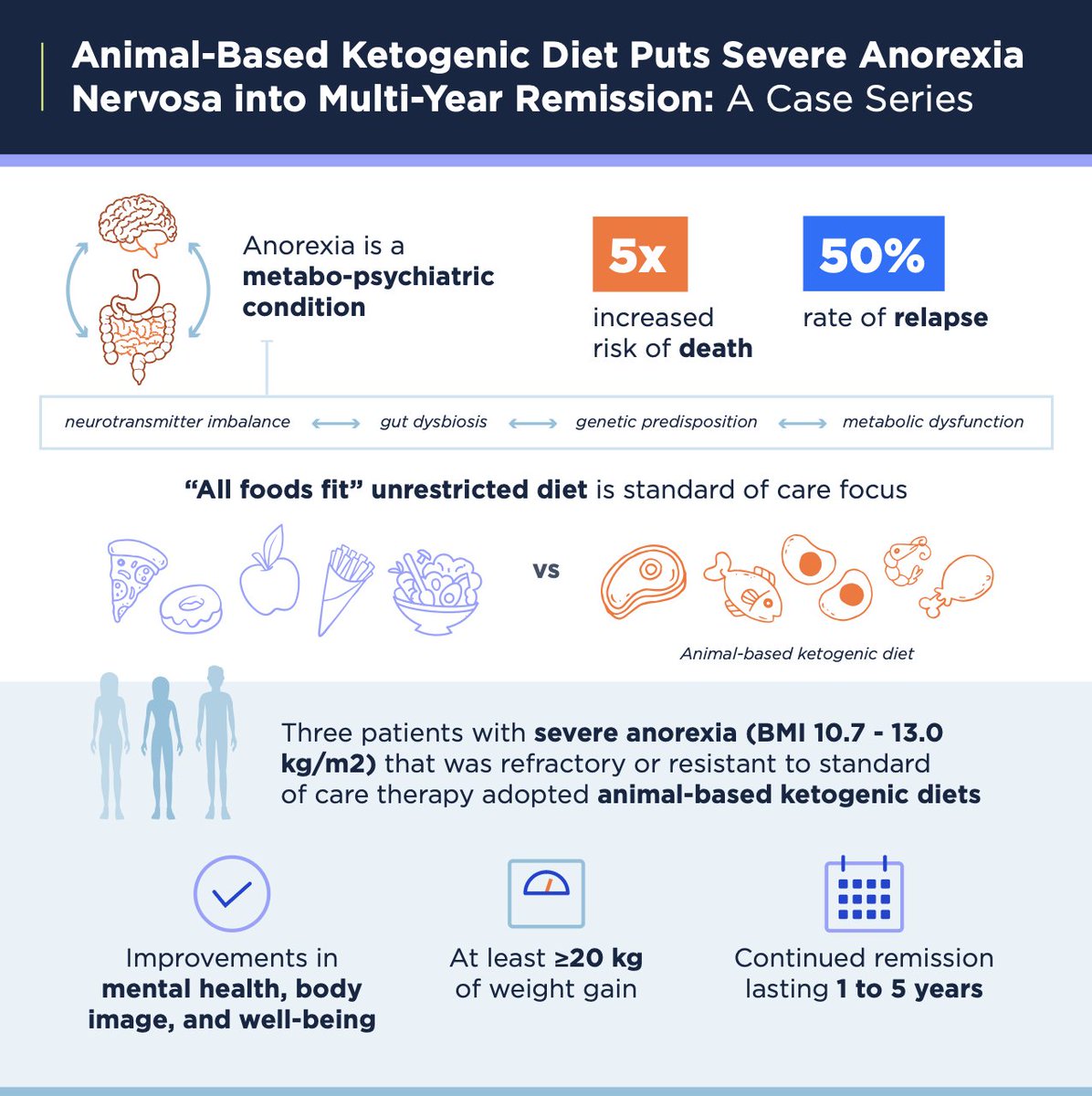
2/10) Background 👇
Anorexia is a devastating condition that increases risk of death >5X and is associated w/ high rates of relapse
There is desperate need for more effective treatment options
Anorexia is a devastating condition that increases risk of death >5X and is associated w/ high rates of relapse
There is desperate need for more effective treatment options
3/10) Common knowledge posits patients w/ anorexia should be discouraged from practicing food group restriction
But anorexia can be framed metabo-psychiatric condition that may benefit from treatment w/ metabolic health interventions w/ neuromodulatory properties, i.e. #ketodiet
But anorexia can be framed metabo-psychiatric condition that may benefit from treatment w/ metabolic health interventions w/ neuromodulatory properties, i.e. #ketodiet

4/10) In this case series, we report on 3 patients who -- after having little success with conventional approaches -- went into remission with an animal-based #ketogenic / #carnivore diet 

5/10) Patient 1 (female):
👉BMI low 10.7 kg/m2
👉 complicated by starvation hepatitis, osteoporosis, anorexia-induced blindness, and cardiac arrest
👉 Quote: "My high-fat #carnivore diet saved me, and I feel I can now do anything. I'm never going back to the way I was"
👉BMI low 10.7 kg/m2
👉 complicated by starvation hepatitis, osteoporosis, anorexia-induced blindness, and cardiac arrest
👉 Quote: "My high-fat #carnivore diet saved me, and I feel I can now do anything. I'm never going back to the way I was"

6/10) Patient 2 (male):
👉BMI 13
👉 complicated by anxiety, low T, neuropathy, osteopenia
👉 Quote: "But when I started a carnivorous diet, my life changed! My anxiety diminished... I steadily gained weight... I'll never go back."
👉Total testosterone levels ⬆ 6X & free T ⬆ 10X
👉BMI 13
👉 complicated by anxiety, low T, neuropathy, osteopenia
👉 Quote: "But when I started a carnivorous diet, my life changed! My anxiety diminished... I steadily gained weight... I'll never go back."
👉Total testosterone levels ⬆ 6X & free T ⬆ 10X
7/10) Patient 3 (female):
👉BMI low 11.8 kg/m2
👉 Complicated by OCD, depression, self-harm
👉 Quote: "I feel 100% in remission and confident it will stick.”
👉Suffered for 3 decades with treatment-resistant anorexia, before starting #ketogenicdiet; now in remission for > 5 years
👉BMI low 11.8 kg/m2
👉 Complicated by OCD, depression, self-harm
👉 Quote: "I feel 100% in remission and confident it will stick.”
👉Suffered for 3 decades with treatment-resistant anorexia, before starting #ketogenicdiet; now in remission for > 5 years
8/10) This case series suggest #ketodiet may have clinical utility for some patients with treatment-resistant anorexia, consistent w/ the perspective of eating disorders as “metabo-psychiatric” conditions that can benefit from neuromodulatory interventions, including ketosis
9/10) We hope these cases inspire further research and attract funding for much-needed clinical trials for ketogenic diets for a variety of mental health conditions, including eating disorders. 

10/10) YOU can help support this line of research by RETWEETING this thread and sharing the link to the paper on your social media share this thread LINK: insulinresistance.org/index.php/jir/…
Special thanks to @Metabolic_Mind, @bschermd, @janellison and the Bazucki Group for their support
Special thanks to @Metabolic_Mind, @bschermd, @janellison and the Bazucki Group for their support
• • •
Missing some Tweet in this thread? You can try to
force a refresh