
In this thread, I will be looking back at highly cited (over 1000 citations) 'historical' (prior to 2008) bionano papers, in particular those that deal with nanoparticles & cells. Comments most welcome. #bionano #NanoBubble
The set of 31 papers considered was identified as follows: WoS search for "Nanoparticle*" [1900-2008, only articles and letters] => 75,260 results of which 181 have over 1,000 citations.
Out of these 57 have a bio angle, e.g. DNA, corona, tumour, cells, etc
Out of these 57 have a bio angle, e.g. DNA, corona, tumour, cells, etc
Some word clouds from those 57 abstracts and author lists (the search term, i.e. "nanoparticle", has been removed from the abstracts word cloud). 



Of the 57, I concentrate on 31 which have something to do with cells; so, eg in vitro ultrasensitive tests are not considered. The thread will build up over several days/weeks - bear with me; or better, join in the fun!
#1, 1998, Bruchez et al “Semiconductor Nanocrystals as Fluorescent Biological Labels" dx.doi.org/10.1126/scienc…
Fig 1 compares spectrum of fluorescein vs one QD spectrum to show the advantages in terms of illumination conditions.
Fig 2 is illustration of size-dependent spectrum.
Fig 1 compares spectrum of fluorescein vs one QD spectrum to show the advantages in terms of illumination conditions.
Fig 2 is illustration of size-dependent spectrum.
Fig 3 is the entire imaging dataset supporting the claim that Qds are promising biological labels. It shows dual colour imaging of nucleus and actin... single image of two cells. It would be worth comparing to standards of the time but not particularly impressive. 

Fig 4 is a photostability graph. Shows that QDs are more stable than fluoroscein.
In short, extremely little data in that paper that reads almost more like an advert than a scientific paper, but of course QDs in bio were super new at the time.
In short, extremely little data in that paper that reads almost more like an advert than a scientific paper, but of course QDs in bio were super new at the time.
#2 Gref et al “Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption”
dx.doi.org/10.1016/s0927-…
dx.doi.org/10.1016/s0927-…
Contrast with #1 is not just in the length of the title (!!!)
Lots of data. Fig 1 shows that PEG reduces protein adsorption (total amount). Fig 2 shows 2D gels (SDS PAGE) of adsorbed plasma proteins; more insights with some proteins reduced at different PEG lengths.
Lots of data. Fig 1 shows that PEG reduces protein adsorption (total amount). Fig 2 shows 2D gels (SDS PAGE) of adsorbed plasma proteins; more insights with some proteins reduced at different PEG lengths.
Figs 3 & 4 do the same thing but looking at the effect of PEG grafting density.
Fig 5 looks at cell uptake (polymorphonuclear cells) and shows that it is prevented by PEG.
An interesting paper.
Fig 5 looks at cell uptake (polymorphonuclear cells) and shows that it is prevented by PEG.
An interesting paper.
Note that they use 'corona' to refer to the polymer shell, NOT to the adsorbed proteins. They characterise quite precisely what would later be known as "the protein corona"... 

#3 2000, Lewin et al “Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells”
doi.org/10.1038/74464
Well, CoI declaration: we are still trying to do this...
Very impressive claims, including detection of a single cell by MRI.
doi.org/10.1038/74464
Well, CoI declaration: we are still trying to do this...
Very impressive claims, including detection of a single cell by MRI.
"CLIO-Tat particles contained an average of four neo-Tat peptides covalently attached to the aminated dextran coating (Fig. 1)" Mat & methods even say 4.1 TAT per particle. Not a critical point but such precision is hard to believe. Data not shown.
No EM, DLS or fluorescence spectrum.
"Punctuate cytoplasmic staining in association with significant nuclear staining was seen after 24 h"
One cell shown (left below). I don't believe that the particles significantly go to nucleus; maybe crosstalk or degradation?
"Punctuate cytoplasmic staining in association with significant nuclear staining was seen after 24 h"
One cell shown (left below). I don't believe that the particles significantly go to nucleus; maybe crosstalk or degradation?

Fig 4 shows that at the concentrations used, CLIO-TAT do not affect the cells biological properties (differentiation). And indeed, the data with or without particles are *incredibly* similar. Near perfect overlap for most data points. 

#4 2002, Åkerman et al “Nanocrystal targeting in vivo”
Isn’t that a short and sweet and simple title? Yes, it is. And that is the message too: that nanocrystals can be used for molecular targeting in vivo.
dx.doi.org/10.1073/pnas.1…
Isn’t that a short and sweet and simple title? Yes, it is. And that is the message too: that nanocrystals can be used for molecular targeting in vivo.
dx.doi.org/10.1073/pnas.1…
What about the evidence? Fig 1 is a scheme of a mouse being injected some particles. Fig 2 is an experiment showing specific binding in cells in culture. Fig 3-5 are ex vivo imaging of tissues after injection of particles.
Fig 3: "In vivo targeting of qdots to normal lung vasculature is specific". Animal is sacrificied 5 min after injection. No image quantification; potential 4bias is large. To the best of my knowledge, the lung-targeting GFE peptide has not been used for imaging be4 nor after. 

Fig 4: "In vivo targeting of qdots to tumor vasculature is specific." Sacrificied 20 min after injection. No image quantification either nor any other independent experimental assessment of targeting. 

Fig 5 shows that PEG coating reduces uptake by the reticuloendothelial system. Again, based on a few selected images.
This paragraph does note some limitations. One wonders why the fluorescein images were not shown for comparison. 

I think some of the success of this paper is not so much due to how impressive the data were, but the short title and promising vision summed up in this concluding sentence 👇
"Although the current nanosystems are rather simple, in the future we envision the fabrication of multifunctional nanosystems, known as nanomachines. Such devices may, eg, sense the presence of disease, deliver a drug 2the site of disease, and release the drug at that site."
#5 2003, Hirsch et al “Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance"
dx.doi.org/10.1073/pnas.2…
The article introduces this simple attractive equation:
non toxic IR + non toxic nanoshells = killing cancer cells
dx.doi.org/10.1073/pnas.2…
The article introduces this simple attractive equation:
non toxic IR + non toxic nanoshells = killing cancer cells
Fig 1 shows the extinction spectrum of nanoshells in water; confirms the overlap of peak nanoshell absorbance with the 820 nm laser. Fig 2 shows that laser kills (and make permeable) the cells only when they have been incubated with the nanoshells. 

No measurement of uptake. Interesting that effect is so big given that the shells are PEG covered (PEG 5,000) therefore low uptake wld be expected.
The rest of the paper shows heating + tissue damage from IR irradiation of a xenograft tumour in which nanoshells hve been injected.
The rest of the paper shows heating + tissue damage from IR irradiation of a xenograft tumour in which nanoshells hve been injected.
#6 2003, Lai et al “A Mesoporous Silica Nanosphere-Based Carrier System with Chemically Removable CdS Nanoparticle Caps for Stimuli-Responsive Controlled Release of Neurotransmitters and Drug Molecules”
dx.doi.org/10.1021/ja0286…
dx.doi.org/10.1021/ja0286…
Last sentence of the abstract "We envision that this new [mesoporous silica nanosphere-based] system could play a significant role in developing new generations of site-selective, controlled-release delivery nanodevices."
The first two figures are schemes showing what they want to make (or what they believe they have made?): a mesoporous system that is closed off by nanoparticles which can then be removed with a chemical trigger allowing stuff inside to diffuse out. 

The nanoparticles (that cap the channels) are CdS quantum dots.
The chemical trigger is DTT or mercaptoethanol cleaving a disulphide bond.
The "stuff" inside is "drug" on the scheme; vancomycin or ATP in experiments.
The chemical trigger is DTT or mercaptoethanol cleaving a disulphide bond.
The "stuff" inside is "drug" on the scheme; vancomycin or ATP in experiments.
The characterization of presence and location of the QDs in the mesoporous materials is shown in Fig 3 and 4. Fig 3 shows two mesoporous particles, one capped with QDs (d, f) and one not capped (c, e). I find it a little hard to reconcile the EM 👇and the cartoon 👆, but... 
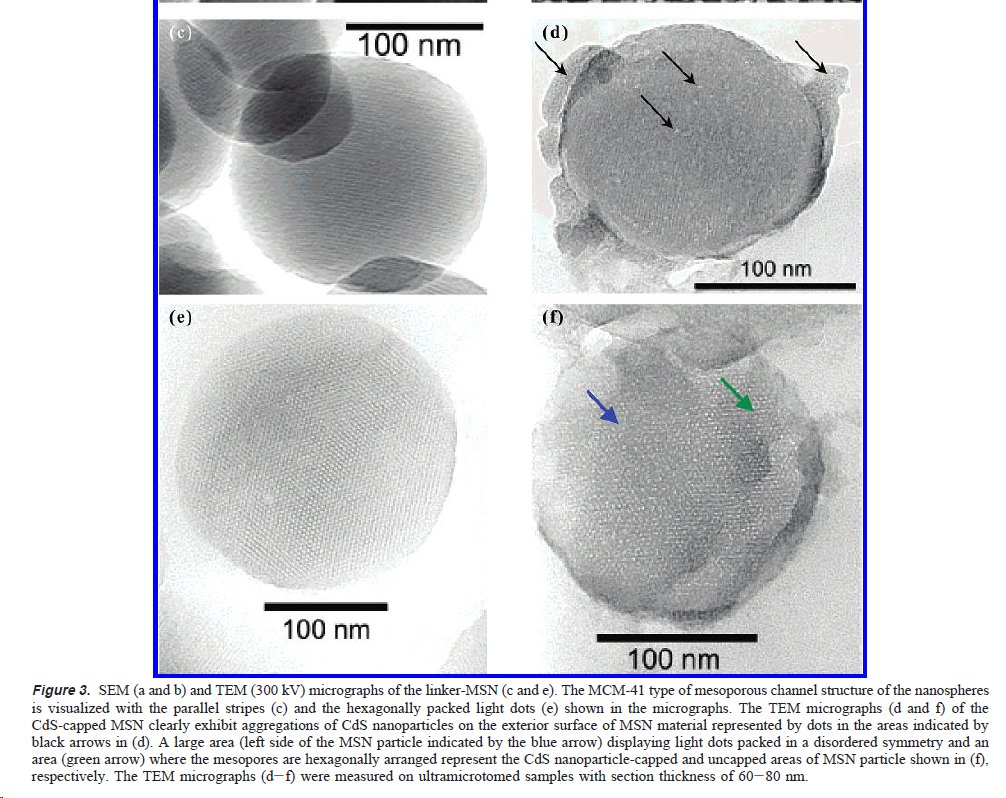
Fig 4 confirms by XRD that CdS QDs are present in the composite materials (though it does not tell us where). How was it possible to close all of the holes of the porous material? Anyway. The controlled release by DTT works (Fig 5); 1mM is necessary to release ~50% after 24h 

There are a couple of controls missing in that Fig 5 though. E.g. Do you get ATP or vancomycin trapped even without the QDs? and if so would it be released by DTT? And are the QDs actually released too?
Finally, we are getting to the experiments with cells...
Finally, we are getting to the experiments with cells...
and it is hard to know where to start with this fig 6... It took me some time to get my head around it. More on this tomorrow though as I need to take a break; watch this space, or even better, let me know what you think of Fig 6! 

OK no one has volunteered comments, so let me start. First, although the abstract talks about the experiments demonstrating "biocompatibility" of the materials, in fact there is no contact with the cells. There are "piles" [their words] of mesoporous nanospheres & cells around.
In Fig 6a and c 👆, we are supposed to see that "perfusion applicatn of ME (1 mM for 5 min) resulted in drastic decrease in fluo intensity of CdS at the ATP-loaded MSN piles (MSN-1 & MSN-2) indicating the CdS caps have been released & diffused away frm the surface-bound MSNs."
But, no, 6a is pseudo colour => no info about intensity.
Only MSN-1 is shown in 6c. Whether it is “drastic” is highly debatable given that y scale is arbitrary and that on the long term it gets back nearly to the initial value...
Only MSN-1 is shown in 6c. Whether it is “drastic” is highly debatable given that y scale is arbitrary and that on the long term it gets back nearly to the initial value...
Why ME instead of DTT? And, why 5 min, 1mM?
The characterization of release (with DTT) in Figure 5b shows that, after 24 hours (!), only 50% release is at that concentration.
The characterization of release (with DTT) in Figure 5b shows that, after 24 hours (!), only 50% release is at that concentration.
OK, so from the above, you can see I am not convinced the data show that the CdS QDs ("the caps") are released. If you have followed, the release of the cap should trigger release of ATP. Here is what the authors say: 

So do the data really show that release of ATP results in a "pronounced increase in intracellular Ca2+"?
No. The upward trends in the red & blue curves exist be4 the supposed release. + that upward trend is also present in the black curve (after the initial "release").
No. The upward trends in the red & blue curves exist be4 the supposed release. + that upward trend is also present in the black curve (after the initial "release").
Although that last point is somewhat moot: I do not understand how the black curve (fluo intensity) can be plotted on the same Y axis as the red and blue curve (ratiometric measurement). The methods section is not helpful in that regard.
To wrap up on that paper: an original (ie hard-to-believe) mechanism for triggered delivery backed by in vitro data with essential controls missing and experiments with cells (not "in cells") which make no sense.
Hypothesis: Lai et al is highly cited because of this sentence "We envision that this new MSN system could play a significant role in developing new generations of site-selective, controlled-release delivery nanodevices."
#7 2003, Wu et al, "Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots”
dx.doi.org/10.1038/nbt764
Now, this one is a sort of follow up, 5 years later, of #1 in this thread. Last author of #7 is first author of #1.
dx.doi.org/10.1038/nbt764
Now, this one is a sort of follow up, 5 years later, of #1 in this thread. Last author of #7 is first author of #1.
#1 announced titles "Semiconductor Nanocrystals as Fluorescent Biological Labels"; 5 years later, #7 continues to make that case w/ more data, focusing on the immunofluorescent labelling of cells.
#7 is accompanied by a News and Views which declares that (title) "Quantum dots finally come of age"
doi.org/10.1038/nbt010…
doi.org/10.1038/nbt010…
#7 is composed of 4 figures (1-4) demonstrating immunolabelling with QDs (Her 2, tubulin and nuclear antigens) and two more figures showing the advantage of QDs over Alexa dyes in terms of signal to noise and photostability.
I have no particular comments on these figures. They are fine & do demonstrate that this kind of things are possible.. but, 16 years later, in hindsight, one can say that QDs did not come of age. Even for that type of applications, i.e. in vitro immuno fluorescence on fixed cells
they have not made any impact on the day-to-day life of biologists/microscopists. I am sure that thousands of papers describing that kind of experiments are published every week and I would bet that the fraction of those using QDs is well below 1%.
Our own recent attempt at using QDs for immunofluorescence has concluded that they have strong limitations:
doi.org/10.3762/bjnano…
doi.org/10.3762/bjnano…
#8 2004, Gao et al “In vivo cancer targeting and imaging with semiconductor quantum dots”
doi.org/10.1038/nbt994
We are staying with quantum dots, but with an application immensely more challenging that immuno fluorescence in fixed cells...
doi.org/10.1038/nbt994
We are staying with quantum dots, but with an application immensely more challenging that immuno fluorescence in fixed cells...
Fig 1 is a scheme of the QDs and a cartoon explaining passive ("EPR effect") and active targeting.
Fig 2 shows targeting of cells with antibody labelled QDs. Top line shows the target being targeted... Second line is the same cell line but QDs without the Ab. [continued]
Fig 2 shows targeting of cells with antibody labelled QDs. Top line shows the target being targeted... Second line is the same cell line but QDs without the Ab. [continued]

The third line shows the QD-Ab particles but with cells which do not express this target.
The problem with this figure is that the contrast is not set in the same way for all three fluo images: the black background is quite different in all three images. Also, no quantification.
The problem with this figure is that the contrast is not set in the same way for all three fluo images: the black background is quite different in all three images. Also, no quantification.
Fig 3 shows "histological examination in six different normal host organs and in C4-2 tumor xenografts maintained in athymic nude mice."
Fig 4 shows some xenograft tumour imaging in a mouse. Quite convincing that there are QDs in the xenograft, and nice use of spectral unmixing (must have been pretty novel at the time?). 

Fig 5 compares targeting by "3 types of surface modifications: COOH groups, PEG groups and PEG plus PSMA Ab". Gao et al concludes that "active tumor targeting by using a tumor-specific ligand is much faster and more efficient than passive targeting based on tumor EPR." 

I am not convinced: no quantification. The main difference between the animal in the centre and the one on the right is that the tumours of the right animal are much bigger. This has nothing to do with differences in the nanoparticle probe...
#9 2004, Sondi et al “Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria”
doi.org/10.1016/j.jcis…
1st sentence of the intro: “Nanosized inorganic particles, of either simple or composite nature, display unique physical...
doi.org/10.1016/j.jcis…
1st sentence of the intro: “Nanosized inorganic particles, of either simple or composite nature, display unique physical...
and chemical properties and represent an increasingly important material in the development of novel nanodevices which can be used in numerous physical, biological, biomedical, and pharmaceutical applications”.
🤔I think I have read this a few times since (possibly before too)
🤔I think I have read this a few times since (possibly before too)
In the case of colloidal silver, this is even true. More on this later.
Figs 1-2 are characterization of the silver nanoparticles (TEM, spectra, size distribution).
Figs 3-4: inhibition of bacterial growth on agar plates; Fig 5: effect is much more limited for solution growth.
Figs 1-2 are characterization of the silver nanoparticles (TEM, spectra, size distribution).
Figs 3-4: inhibition of bacterial growth on agar plates; Fig 5: effect is much more limited for solution growth.
Fig 5-9 are SEM, TEM and EDAX data showing that nanoparticles interact with the membranes. Some of it is nice eg Fig 8, some harder to interpret eg Fig 9 (+1 for diplomacy here, right?). 



Overall, a reasonable paper with claims largely backed by data... but.
Yes, there is a "but", and one I find particularly interesting (@ccmmody might like this too).
Yes, there is a "but", and one I find particularly interesting (@ccmmody might like this too).
Their claim to fame is “To our knowledge, the antibacterial activity of silver ions is well known and has been studied in detail [19–21], while the antibacterial activity of nontoxic elementary silver, in the form of nanoparticles, has not been reported in the literature.”
Maybe technically right, ie their knowledge was very deficient. In 2004, Acticoat, a dressing using colloidal silver (ie nanoparticles) was used in clinical practice. Still used:
smith-nephew.com/key-products/a…
Paper published 5 years before #9:
doi.org/10.1097/000046…
smith-nephew.com/key-products/a…
Paper published 5 years before #9:
doi.org/10.1097/000046…
Colloidal silver had been used extensively as an antibacterial nearly a century (!) before #9.
doi.org/10.12968/bjcn.…
doi.org/10.12968/bjcn.…
"MacLeod (1912) refers to his experience w/ colloidal silver — Collosol
Argentum — introduced by Crookes Chemists in 1911 4the treatment of bacterial infections by topical, intravenous, hypodermic &oral dosage"
Argentum — introduced by Crookes Chemists in 1911 4the treatment of bacterial infections by topical, intravenous, hypodermic &oral dosage"
#10 2005, Connor et al "Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity"
doi.org/10.1002/smll.2…
It is a communication, two figures in ms + 1 in supporting info, but I can't find the SI (can someone help me?).
doi.org/10.1002/smll.2…
It is a communication, two figures in ms + 1 in supporting info, but I can't find the SI (can someone help me?).
Rationale: "Nanosci &nanotech hold great promise 4many applications, incl biomedical uses. Yet despite the huge potential benefit [.], very little is known about potential short- and long-term deleterious effects of such nanomaterials on human and environmental health."
Figure 1 shows that cells are killed by gold chloride and by CTAB, i.e. stuff used in the synthesis and modifications of nanoparticles, but not by the nanoparticles themselves. 

Figure 2 shows that nanoparticles do get inside the cells, by TEM and by reduction of the absorbance of the cells medium. That is all fine. 

But, like with #9, there is an ignorance of things that had been well known a long time ago. Authors justify need for fig 2 as follows: "The lack of detectable cytotoxicity raised the question of whether the nanoparticles were capable of being taken up into the cells."
Gold colloids uptake into cells has been used in the 1950s and 1960s as a model to understand how cells probe their environment and how viruses penetrate cells, e.g.
dx.doi.org/10.1083/jcb.3.…
and ncbi.nlm.nih.gov/pubmed/14164483
dx.doi.org/10.1083/jcb.3.…
and ncbi.nlm.nih.gov/pubmed/14164483
The oldest reference in #10 is from 2001.
@threadreaderapp unroll please!
• • •
Missing some Tweet in this thread? You can try to
force a refresh









