😮! These findings significantly expand on a central mechanism by which persistent #pathogens drive chronic disease 👉 Namely, the ability of intracellular pathogens to dysregulate host receptor actvity in a manner that alters downstream pathway signaling: stke.sciencemag.org/content/12/599… 



2/ Specifically they found that a “vast number” of intracellular pathogens can bind (or create #proteins that bind) #receptor tyrosine kinases (RTKs) on the surface of host cells 👉 This facilitates their entry into the host cell + dysregulates cell signaling in numerous ways. 

3/ Look at the figure below, which lists dozens + dozens of human #viruses with this capacity 👉 These viruses can dysregulate RTK activity to facilitate their entry into host cells by clustering in #lipid rafts, adjusting cytoskeletal dynamics, modulating endocytosis etc etc 
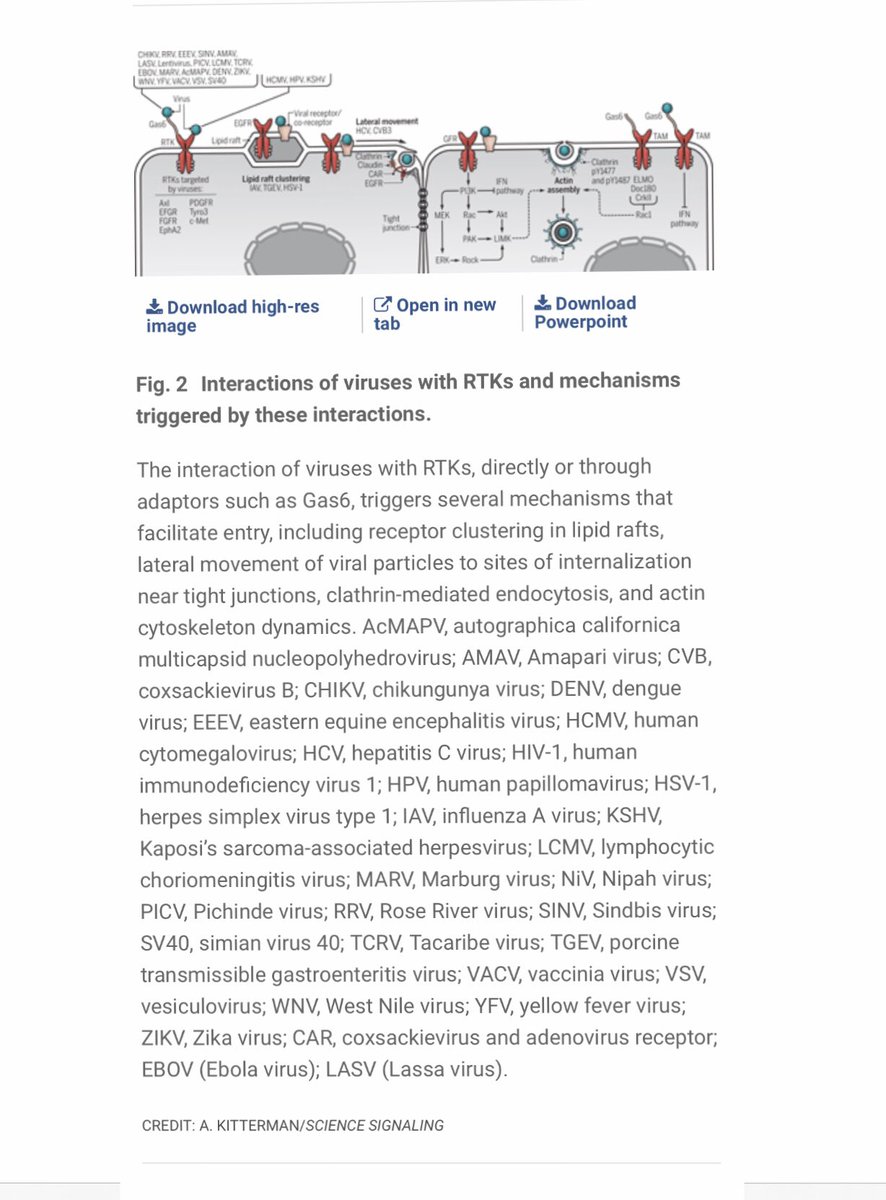
4/ Interestingly, already existing cancer #drugs that target RTKs might be able to be repurposed to block #pathogens from being able to enter/dysregulate human cells via this mechanism: google.com/amp/s/medicalx…
• • •
Missing some Tweet in this thread? You can try to
force a refresh










