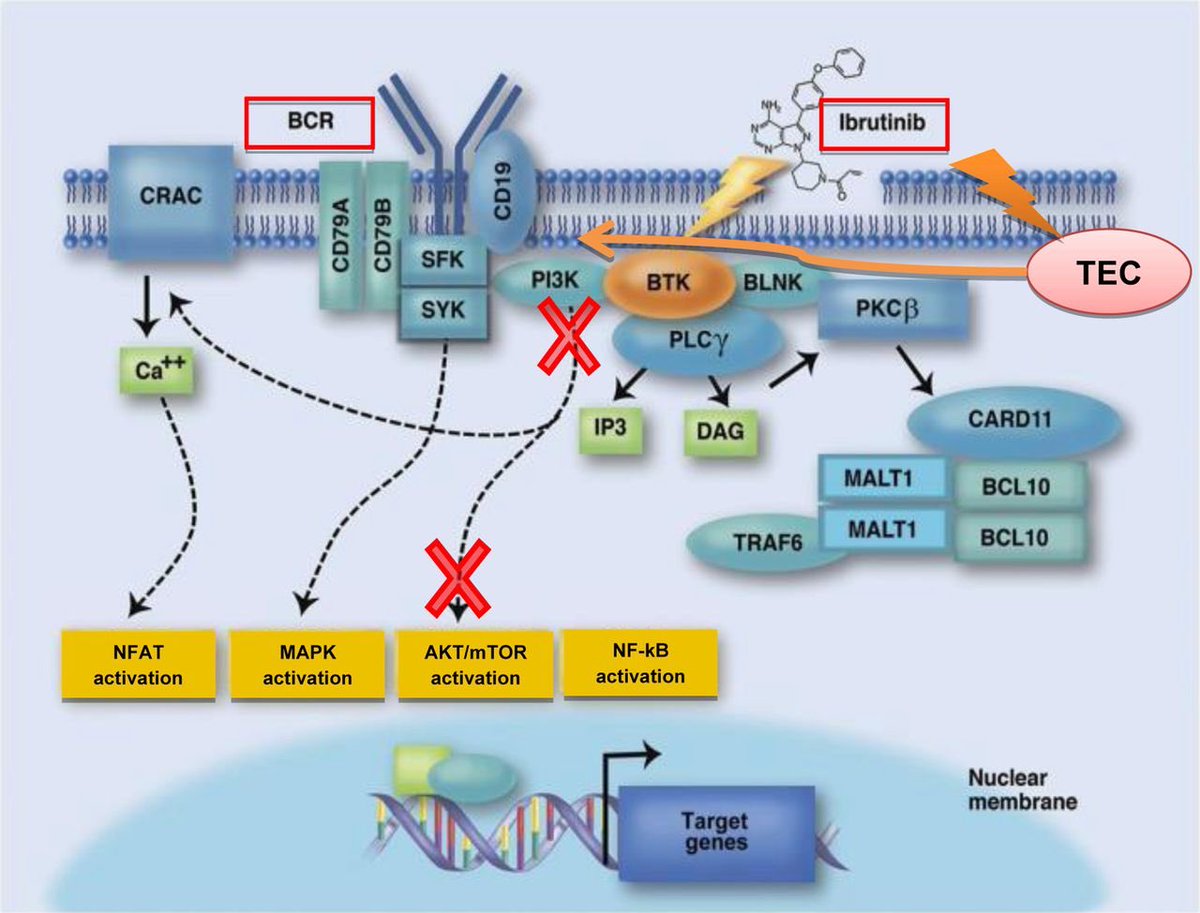
Okay for all those non-oncology experts walking into this situation, let’s take a few steps back 🦶 and give a background on ibrutinib 👩🏫📜
✅improved progression-free survival, ✅overall response rates, and ✅ overall survival
This was compared w/ ofatumumab (AKA rituximabs cousin) & ⚠️ chlorambucil ⚠️
HOWEVER as @PamelaPLN mentioned, studies assessing patients with longer follow up (28 months) the incidence rose as high as 16% 😟
BTK is expressed in ❤️ tissue & ibrutinib has off target effects on other kinases in cardiac tissue that play a role in maintaining sinus rhythm Therefore, it is plausible that ibrutinib ↗️ vulnerability to AF by ⬇️regulation of these pathways
Let’s first take a dive into anticoagulation...
Traditional risk scoring tools to assess the need for AC (CHADS2VASC) have NOT been validated in cancer patients
Can I get a #cardioonc consult on this 😅
&to make things more complicated
ibrutinib downregulates glycoprotein expression on platelets which ⬇️ adhesion to von willebrand 👉🏻 ↗️ bleeding risk even w/o AC (~up to 44%!) & risk is only further↗️ when AC is required🤯
Ibrutinib: CYP3A4 substrate, P-gp inhibitor
❌Dabigatran (p-gp substrate)
✅rivaroxaban & apixaban
⚠️Warfarin (N/V & ⬇️oral intake can lead to alterations in INR and↗️bleeding: less preferred)
Initial ⬇️ dose followed by approved maintenance doses based on tolerability has been suggested
BUT does not account for ↗️ bleeding risks when using full therapeutic doses
jhoponline.com/jhop-issue-arc…
1st for rate control:
✅BB preferred
❌Diltiazem & verapamil👉🏻 potent CYP3A4 inhibitors ↗️ ibrutinib toxicity
On the other hand ibrutinib ↗️
❌Digoxin concentrations through 🚫 of p-gp substrate
Pearl: controlling HTN with BB can help ⬇️ risk of AF! Thanks @PharmDickerson ashpublications.org/blood/article-…
Amiodarone is fraught by many DDI with both anticoagulants and nodal blocking agents and based on the above noted info, would increase concentrations of ibrutinib for as long as amio remains in the system (~60 days 😅)