THREAD
Data from Birmingham garnered LOTs of negative press about #COVID19 rapid Ag tests
The data
1) is very small, 8 positives
2) Shows EXACTLY what we expect, Rapid Ag tests detect infectious ppl, not super low PCR RNA
Overall, the conclusions IMO SUPPORT rapid tests
1/
Data from Birmingham garnered LOTs of negative press about #COVID19 rapid Ag tests
The data
1) is very small, 8 positives
2) Shows EXACTLY what we expect, Rapid Ag tests detect infectious ppl, not super low PCR RNA
Overall, the conclusions IMO SUPPORT rapid tests
1/
https://twitter.com/alanmcn1/status/1342772827112542216
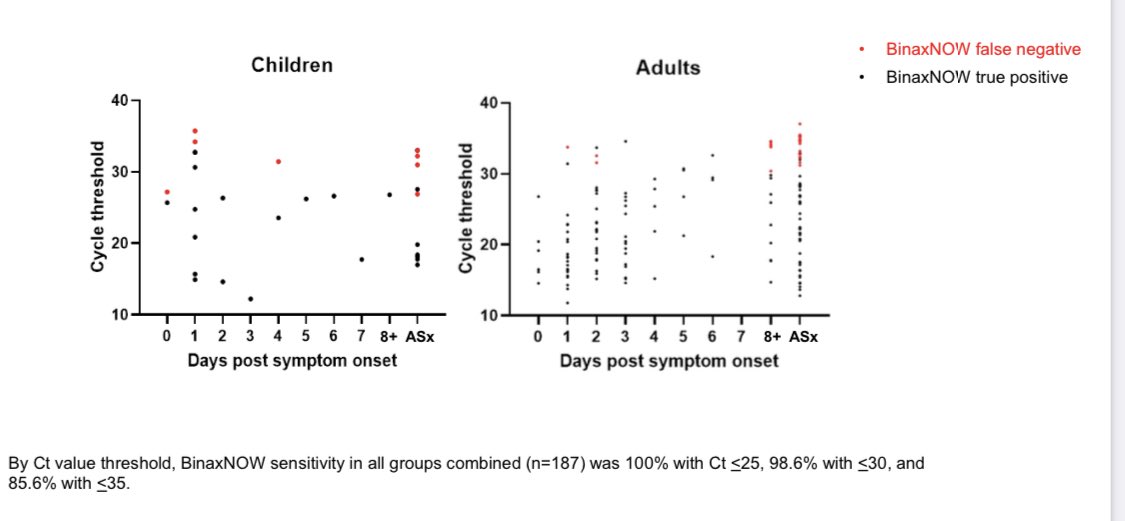
We know that high PCR Ct values (very low RNA) above 30 are generally not culturable/not likely contagious
For context, a Ct value of 30 is ~99.999% lower RNA than peak RNA when most contagious.
The Rapid tests in the study appear to have a limit of ~30
2/
For context, a Ct value of 30 is ~99.999% lower RNA than peak RNA when most contagious.
The Rapid tests in the study appear to have a limit of ~30
2/
So, even when virus is culturable at Ct 30, it is much lower amt than peak virus titers/when most transmissible.
Plus, Missing a Ct of 30+ is most likely missing someone AFTER peak viral load and not before Bc the time spent around 30-35 is much longer after than before.
3/
Plus, Missing a Ct of 30+ is most likely missing someone AFTER peak viral load and not before Bc the time spent around 30-35 is much longer after than before.
3/
But the real issue with the study is that it is based on only 8 positive samples. 6 were 29-35. 2 were low or mid 20’s.
As expected, the rapid tests caught the high viral loads and didn’t catch the very low RNA loads. That we know were most likely late/post-infection.
4/
As expected, the rapid tests caught the high viral loads and didn’t catch the very low RNA loads. That we know were most likely late/post-infection.
4/
This is the whole point of rapid tests. Test frequently with rapid results to catch infectious people before they spread to others.
For public health screening, we are not trying to medically diagnose recent infections. We are trying to isolate contagious people.
5/
For public health screening, we are not trying to medically diagnose recent infections. We are trying to isolate contagious people.
5/
Overall, the paper described it in a fairly balanced way. Could have discussed more about what we know of high Cts and cultureability.
The numbers were too low and led to unstable estimates:
Ie sensitivity
100% w Ct <29
9% w Ct <= 29
Thus serious problems w sample size.
6/
The numbers were too low and led to unstable estimates:
Ie sensitivity
100% w Ct <29
9% w Ct <= 29
Thus serious problems w sample size.
6/
Overall, results align with our expectations of rapid antigen tests. Even in this extremely limited study, they demonstrated similar results to many other studies & show again that these tests, when used frequently can potentially be very powerful tools to slow transmission.
7/7
7/7
• • •
Missing some Tweet in this thread? You can try to
force a refresh