
Writing a column, 12-15 00 words is never easy. especially if you want to be factual, comprehensive, fair, and logical. And especially if you want it to be read widely. I faced this difficulty in rising to the challenge posed by @1amnerd when he invited me to explian this: 

This was the Lancet paper of 21 Jan from an @BharatBiotech and @ICMRDELHI team reporting their phase 1 trial of BBV152 (#Covaxin). Ordinarily, a technical paper of this kind would excite interest only among PhDs and others in the same field of science.
The reason there was a story in it at all was that it was widely misrepresented on both social and mainstream media. The subliminal suggestion was that the 03 jan decision to authorise #Covaxin was after all vindicated. 






Mere mortals wouldn't give a monkey's about the technical minutiae of CD4/CD8 and IgG1/G4 ratios. Who cares what an RBD is? (it is a Receptor Binding Domain, if you really must know). Charts like this are not designed to excite, are they? 

The mainstream media took the easy route, It was a much easier story to report if you took the line that here at last was a prestigious foreign journal finally succumbing to the sheer weight of science and acknowledging the Indian breakthrough by publishing the results.
But Indian science stands up to the best in the world. Indian scientists have a well-earned reputation for frugal innovation. If they are let down at all it is not by the studied indifference of Western journals but by needless homegrown jingoism. science.thewire.in/health/dcgi-cd…
Explaining this takes domain knowledge, a love for writing, and an ability with language. Too technical loses you readers, too simple loses you scientific accuracy. There's no right or single middle path. Too critical and the pseudo-national trolls latch on like piranhas
It helps to have a good editor who understands the readership. But even before it got to that stage, I had rapidly to catch up with the advances in immunology that had, over the years, passed me by. In the final finished article as it appeared on the web: science.thewire.in/the-sciences/c…
And so it was gratifying to have a medical scientist like @chatterbox7916 not only read the article but read it closely enough to spot an error. This line "The other kind of T-cell mediated immune response, Th-2, is associated with cytokines.", was wrong, misleading she said.
And true enough, it was misleading. Both Th1 and Th2 immune responses are mediated by cytokines. What are cytokines and what are Th1 and Th2 immune responses. I found this an excellent primer on the subject. bmj.com/content/321/72…
"Cytokines are the hormonal messengers responsible for most of the biological effects in the immune system, such as cell-mediated immunity and allergic-type responses."
"Although they are numerous, cytokines can be functionally divided into two groups: those that are proinflammatory and those that are essentially anti-inflammatory but that promote allergic responses"
Specifically in relation to vaccine development, Soumya Chatterjee referred me to this very helpful and relatively easy to understand paper in PNAS from April 2020. pnas.org/content/pnas/1… 

And I found another nice review paper in Science from May 2020 that gives a thorough account of the need for caution and careful testing to ensure that a vaccine intended to prevent disease does not result in even worse disease. science.sciencemag.org/content/sci/36…
The new terms to come up to speed with, are
#ADE or Antibody-mediated disease Enhancement
#VAERD vaccine-associated enhanced respiratory disease. And here is a nice summary of the risks.
#ADE or Antibody-mediated disease Enhancement
#VAERD vaccine-associated enhanced respiratory disease. And here is a nice summary of the risks.
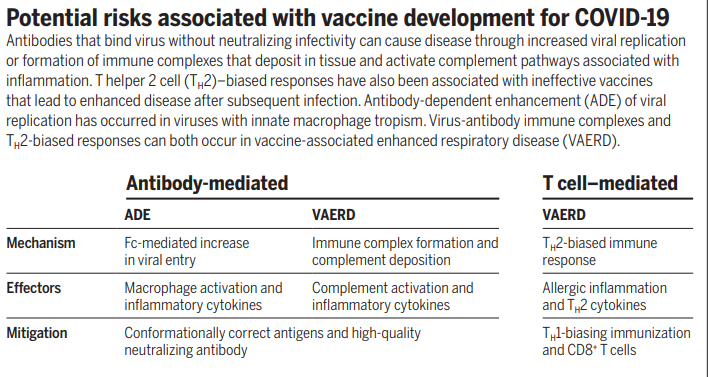
There is far more in these two papers than can be summarised meaningfully in a Tweet thread. If you're interested you'll have to follow the links and read them. But back once one last time to the practical significance of #ADE and #VAERD
We need to proceed carefully, but that does not mean delay. We need effective vaccines, and fast, but cutting corners risks a setback as happened before with other epidemics like SARS. We need to carry the public with us and that means transparency.
END
END
• • •
Missing some Tweet in this thread? You can try to
force a refresh





